Abstracts
Abstract
A survey for detection of barley yellow dwarf luteoviruses (BYDV-PAV and BYDV-MAV), cereal yellow dwarf polerovirus (CYDV-RPV), barley stripe mosaic hordeivirus (BSMV), wheat dwarf monogeminivirus (WDV) and brom mosaic bromovirus (BMV) was carried out during May 2003 covering seven cereal-growing counties of Tekirdag, Turkey. Two hundred sixty (260) wheat samples with yellowing, stunting, or striping were collected from 26 wheat fields. These samples were tested for the presence of six viruses by ELISA using polyclonal antisera. Serological tests showed that six tested viruses were present in Tekirdag, Turkey. Among the tested viruses, BYDV-MAV was the most commonly detected (25% of the 260 wheat samples), followed by BYDV-PAV (22.3%), WDV (16.5%), CYDV-RPV (8.5%), BMV (3.1%) and BSMV (1.5%). This study revealed the presence of six viruses in wheat fields in Tekirdag and reported for the first time BSMV and BMV on wheat in Turkey.
Résumé
Une étude visant à détecter les lutéovirus de la jaunisse nanisante de l’orge (BYDV-PAV et BYDV-MAV), le polérovirus de la jaunisse nanisante des céréales (CYDV-RPV), l’hordeivirus de la mosaïque striée de l’orge (BSMV), le monogeminivirus du nanisme du blé (WDV) et le bromovirus de la mosaïque du brome (BMV) a été effectuée au cours du mois de mai 2003 dans sept comtés céréaliers du Tekirdag, Turquie. Deux cent soixante (260) échantillons de blé présentant du jaunissement, du rabougrissement ou de la striure ont été prélevés dans 26 champs de blé. Ces échantillons ont été analysés par ELISA pour détecter la présence de six virus à l’aide d’antisérums polyclonaux. Les tests sérologiques ont révélé que les six virus testés se retrouvaient au Tekirdag, Turquie. Parmi les virus testés, le BYDV-MAV a été le plus fréquemment détecté (25,0 %) dans les 260 échantillons de blé examinés, suivi du BYDV-PAV (22,30 %), du WDV (16,53 %), du CYDV-RPV (8,46 %), du BMV (3,07 %) et du BSMV (1,53 %). La présente étude a montré l’existence de six virus dans les champs de blé du Tekirdag et signale pour la première fois la présence du BSMV et du BMV sur le blé en Turquie.
Article body
Introduction
Cereal production mainly depends on barley and wheat in Turkey. Cereal production was 29 570 560 tons in Turkey in 2001, with 19 007 000 tons of wheat (Anonymous 2003). Annual wheat production in Tekirdag province was 845 772 tons (Anonymous 2001).
Several viruses may affect wheat crops including barley yellow dwarf viruses (BYDV-MAV, BYDV-PAV, BYDV-RMV, BYDV-SGV, BYDV-GPV), cereal yellow dwarf virus (CYDV-RPV), brome mosaic virus (BMV), wheat dwarf virus (WDV), barley stripe mosaic virus (BSMV), wheat soil-borne mosaic virus (SBWMV), and wheat streak mosaic virus (WSMV) (Conti et al. 1990; Fitzgerald and Stoner 1967; Hofmann and Kolb 1998; Makkouk et al. 1990; McKirdy and Jones 1997; Mc-Kirdy et al. 2002; McNeal et al. 1976; Rochow 1969; Sutic 1999).
The most widely spread and important pathogens of cereal crops include barley yellow dwarf luteoviruses (BYDVs) and cereal yellow dwarf virus (CYDV-RPV) (Conti et al. 1990; Makkouk et al. 1990; McKirdy et al. 2002; Oswald and Houston 1951; Plumb 1983). Several BYDV strains have been differentiated on the basis of vector specificity, serological and molecular properties (Gill 1969; Hammond et al. 1983; Harrison 1999; Miller et al. 2002; Pringle 1999; Rochow 1969, 1979). Among BYDV strains, BYDV-MAV and BYDV-PAV belong to the genus Luteovirus (MAV specifically transmitted by Sitobion avenae (F.) [Homoptera : Aphididae], PAV non-specifically transmitted by both Rhopalosiphum padi (L.) [Homoptera : Aphididae] and S. avenae), the remaining three strains (BYDV- GPV, BYDV-RMV and BYDV-SGV) were classified as unassigned within the family Luteoviridae (Mayo and D’Arcy 1999; Pringle 1999). The name of the BYDV-RPV strain (specifically transmitted by R. padi) was changed to cereal yellow dwarf virus (CYDV-RPV) which belongs to the genus Polerovirus within the family Luteoviridae (Harrison 1999; Miller et al. 2002). The losses and disease severity in wheat crops depend on the sensitivity of the varieties, the virus strains, the time of sowing, the time of infection and the environmental conditions (Gill 1969; McGrath and Bale 1990; McKirdy and Jones 1997). Characteristic symptoms in infected plants are yellowing, reddening and brittleness of leaves. Infected plants express delayed maturity, growth, and dwarfing. Root systems are reduced, heading and filling may be inhibited, and tillering may also be reduced.
Wheat dwarf monogeminivirus (WDV) was first described in Czechoslovakia by Vacke (1961). WDV has been described in Russia, Sweden, Bulgaria, Slovakya and Romania, Hungary, Sweden, France, Morocco and Turkey (Bendahmane et al. 1995; Bisztray and Gaborjanyi 1989; Lindsten et al. 1970; Najar et al. 2000; Pocsai et al. 2003). WDV causes stunting and leaf discoloration on wheat, often more prominent on the older leaves of infected plant. Leaves of WDV-infected wheat usually turn yellow and sometimes red. The virus can cause reduced tillering, sterility and failure to fill kernels. Yield reductions caused by WDV varied from 5 to 97% depending on the time of infection (Vacke 1988). The vector of the virus, Psammotettix alienus Dhb. [Homoptera: Cicadellidae] transmits the virus in a persistent manner (Vacke 1961).
Barley stripe mosaic hordeivirus (BSMV) can infect barley, wheat, oats, maize and numerous grasses (Atabekov and Novikov 1989; Carroll 1980). The virus occurs in the pollen and seed of the majority of gramineous host plants (Bennett 1969). The virus can survive 20 yr in wheat seed (McNeal et al. 1976). Symptom intensities range from a mild stripe mosaic to necrosis, followed by complete dwarfing (Atabekov and Novikov 1989).
Brome mosaic bromovirus (BMV), that was isolated first from brome plants (Bromus inermis Leyss.), infects several gramineous plants (Tosic 1971) belonging to 50 genera of the Gramineae (Lane 1977; Sutic 1999). BMV causes elongated mosaic spots and stripes on wheat leaves, infection early in the season expresses delayed growth and significantly shortened spikes. Nematodes, beetles, mites and the rust fungus (Puccinia graminis Pers.:Pers. f. sp. tritici Eriks. & E. Henn.) are involved in the transmission of the virus (Cooper 1988). The virus is transmitted mechanically with sap of infected plants, which may be important for the natural spread of BMV (Lane 1977).
Wheat streak mosaic virus (WSMV) (Bremer 1971), barley yellow dwarf virus (BYDV) (Bremer and Raatikainen 1975; Makkouk et al. 1994; Pocsai et al. 2003), maize mosaic virus (MMV) (Bremer and Raatikainen 1975), barley yellow stripe virus (BYSV) (Bremer and Raatikainen 1975), and wheat mosaic virus (WMV) (renamed soil-borne wheat mosaic virus, SBWMV) (Köse and Ertunç 1999; Kurçman 1981) diseases were reported in cereal growing areas in Turkey. The aim of this study was to determine the presence of six cereal viruses on wheat grown in Tekirdag, Turkey.
Materials and methods
In May 2003, a survey was carried out for the determination of the incidence rates of BYDVs (BYDV-MAV and BYDV-PAV), CYDV-RPV, WDV, BSMV and BMV in wheat fields in different counties of Tekirdag, Turkey (Fig. 1). Ten plant samples exhibiting yellowing, dwarfing, leaf streaking and mosaic like symptoms were collected from each of 26 selected fields and kept in nylon bags at 4°C in refrigerator. Virus diagnosis was done by DAS-ELISA as described by Lister and Rochow (1979). The antisera and conjugates were purchased from BIOREBA-Switzerland (BYDV-MAV, BYDV-PAV, CYDV-RPV), LOEWE Biochemica-Germany (WDV) and DMSZ-Germany (BSMV and BMV)).
The samples were tested in the plant pathology laboratory, Department of Plant Protection, Tekirdag Faculty of Agriculture, Tekirdag, Turkey. The wheat samples collected were homogenized using a mortar and pestle with the addition of sample extraction phosphate buffer solution (8.0 g NaCl, 0.2 g KH2PO4, 2.9 g Na2HPO4.12H2O, 0.2 g KCl, 0.2 g NaN3, 20 g polyvinylpyrrolidone-25 per L, pH: 7.4) at a ratio of 1:5. Plates were precoated with viruses antisera which were diluted in carbonate buffer (1.59 g Na2CO3, 2.93 g NaHCO3, 0.2 g NaN3 per L, pH: 9.6) and incubated for 2 h at 37°C. After washing the plates with PBST buffer (8.0 g NaCl, 0.2 g KH2PO4, 2.9 g Na2HPO4.12H2O, 0.2 g KCl, 0.2 g NaN3, 0.5 mL Tween-20 per L), samples were added to wells and incubated overnight at 4°C. Alkaline phosphatase conjugated antibody diluted in conjugate buffer (PBST + 2% polyvniylpyrrolidone-25 + 0.2% BSA (bovine serum albumin, Sigma A-4503), pH: 7.4) were added after washing the plates and incubated for 2 h at 37°C. P-nitrophenylphosphate in substrate buffer (97 mL Diethanolamine, 0.2 g NaN3 per L, pH: 9.8) was added to each well and incubated for 2 h at room temperature before absorbance value were read at 405 nm using a Programmable Microplate Reader (Model DV990BV4- GIO. DE VITA E C S.r.l. –Rome, Italy).
Results
Wheat samples showing dwarfing, yellowing, yellow mosaic, stripe mosaic and stunting were collected from randomly selected fields in seven different counties in Tekirdag, Turkey. Ten plant samples were collected from each of the 26 winter wheat fields in May 2003. Dwarfing and yellowing were the most common observed symptoms. Stunted plants often appeared in 1-2 m diam patches, with most severe symptoms in the center and decreasing outwards. Scattered, individually symptomatic plants were also very common. Wheat was severely stunted and exhibited a bright yellow color in many fields. Severe drought has been observed in some fields and caused symptoms similar to those induced by virus infection or excess water stress i.e. total yellowing of plants.
The results showed that 24 of the 26 tested wheat fields were infected with tested viruses (Table 1). BYDV-MAV was found as the most common virus present in 18 out of 26 tested wheat fields, followed by WDV in 17 fields, BYDV-PAV in 13, CYDV-RPV in 10, BMV in 4, and BSMV in 3 (Table 1).
Figure 1
Location of collected wheat samples in Tekirdag province, Turkey.
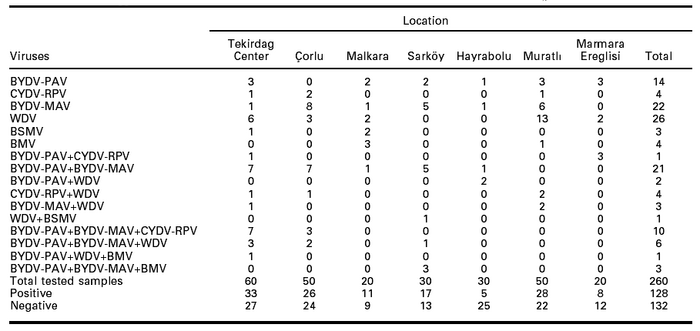
Table 1
Number of virus infected wheat fields in Tekirdag, Turkey in 2003
From the 260 wheat samples tested for presence of six virus infection, our results indicated that BYDV-MAV and BYDV-PAV were the two most widespread viruses, with respectively 25% and 22.3% infection ratio in surveyed area, followed by WDV (16.5%), CYDV-RPV (8.5%), BMV (3.1%) and BSMV (1.5%) (Table 2).
Table 2
Number of virus infected plants in tested wheat samples in Tekirdag, Turkey in 2003
In wheat grown in Tekirdag center, BYDV-PAV was the most widespread virus (with 36.7% of tested plants), followed by BYDV-MAV (31.7%), WDV (20%) and CYDV-RPV (16.7%) (Table 2). BSMV and BMV were both determined only in one sample each (1.7%) Wheat fields presented yellowing of flag leaves and dwarfing; however, dwarfed plants from edge of the fields suggest an early infection while plants with yellowing of flag leaves observed in different part of the fields suggest late infection.
In Çorlu, BYDV-MAV was the most widespread virus (with 40% of tested plants) followed with BYDV-PAV (24%), CYDV-RPV (12%) and WDV (12%) infections (Table 2). Both dwarfing and yellowing were scarcely observed on the plants, except for one field where strong yellowing and dwarfing were present in most of the field.
Interestingly, BMV and BYDV-PAV were determined with 15% infection ratio in Malkara (Table 2). Infection ratio was 10% for BYDV-MAV, WDV and BSMV respectively. Total yellowing of many plants was observed because of flooding in one field, virus infection was also determined in the samples collected from this field. CYDV-RPV was not determined in any samples tested from Malkara.
BYDV-MAV was also found widespread in Sarköy city with 46.7% infection ratio, followed with BYDV-PAV (36.7%), BMV (10%), WDV (6.7%) and BSMV (3.3%) (Table 2). Plants with yellow flag leaf were common, however dwarfed plants were rare. Flooded fields were not tested for virus infection.
Infection ratio was low in wheat samples collected from Hayrabolu (Table 2). BYDV-PAV was determined in 13.3% of the wheat samples, followed by BYDV-MAV and WDV in 6.7%. Discoloration and yellowing of flag leaves were scarcely observed in sample fields.
WDV was the most widespread virus in Muratli with 34% infection ratio (Table 2). It was followed with BYDV-MAV, BYDV-PAV, CYDV-RPV and BMV with respectively 16, 6, 6 and 2% infection ratio. Dwarfed plants and yellowing of flag leaves were observed. Most dwarfed plants were located near the edge of the field.
The only 3 three viruses determined in Marmara Ereglisi were BYDV-PAV, CYDV-RPV and WDV with respectively 15, 15 and 10% of infected plants (Table 2).
Of the 260 tested samples, the infection ratio was almost similar in five of the seven counties (Fig. 2). It was 56.7% in Sarköy, 56% in Muratli, 55% in Tekirdag center, 55% in Malkara and 52% in Çorlu. In Marmara Ereglisi and Hayrabolu counties, infection ratio was 40 and 16.7% respectively.
Regarding the six tested viruses, WDV was the most commonly detected being present in 10% of 260 tested wheat samples (Table 3). BYDV-MAV and BYDV-PAV infected respectively 8.5% and 5.4% of the sampled plants. CYDV-RPV and BMV infection types were determined at lower ratio (1.5%). The most common mixed infection type was BYDV-PAV + BYDV-MAV (8.1% of tested plants) followed with BYDV-PAV + BYDV-MAV + CYDV-RPV (3.8%) and BYDV-PAV + BYDV-MAV + WDV (2.3%). However, very low infection ratio (0.4%) occurred with BYDV-PAV + CYDV-RPV, WDV + BSMV and BYDV-PAV + WDV + BMV.
Interestingly, BMV was only determined in wheat samples from Malkara and Muratli (Table 3). BYDV-PAV + CYDV-RPV double infections were determined in samples from Tekirdag center and Marmara Ere-glisi, BYDV-PAV + WDV in samples from Hayrabolu, BYDV-MAV + WDV in samples from Tekirdag center and Muratli. Multiple infections were also detemined: BYDV-PAV + BYDV-MAV + CYDV-RPV in samples from Tekirdag center and Çorlu, BYDV-PAV + WDV + BMV in samples from Tekirdag center, and BYDV-PAV + BYDV-MAV + BMV in samples from Sarköy. The infection types that occurred are related to the ability of the vectors to acquire and transmit the viruses and to the presence of the surveyed viruses on the weeds and crop host plants.
Figure 2
Infection ratio (%) of tested viruses in wheat samples from seven counties in Tekirdag, Turkey in 2003.
Table 3
Infection types of cereal viruses in tested wheat samples in seven counties in Tekirdag, Turkey in 2003
Discussion
The symptoms were generally characteristic for the tested viruses, however yellowing and dwarfing caused by excess water and nutrient deficiency or drought favored slow growing of the plants. The incidence of virus diseases varies from yr to yr depending on the environmental conditions that favor the vector occurrence and development, susceptibility of the plants and cultural operations in cereal production. The yield losses caused by BYDV infections may reach 9 to 79% depending on the variety of the plants and on infection period (Sutic 1999). During surveys, drastic yield losses have been observed in wheat fields in many yr in Tekirdag. Affected fields showed patches with a few m diam with yellowing and dwarfing of plants. Slow development and dwarfing of infected plants near field borders may have been caused by early infections of the viruses. BYDV-PAV is reported as the most widespread serotype in cereals worldwide (Conti et al. 1990; El-Yamani and Hill 1990; Hofmann and Kolb 1998; Rochow 1979). The results obtained in this field experiment were not different from other cereal growing areas. All tested viruses were determined in wheat samples in different infection ratios. BYDV-MAV seemed the most prevalent virus present in 18 out of 26 tested fields and was widespread in Çorlu region. BYDV-PAV was dominant in samples collected from Tekirdag center. BSMV was only detected in three fields. Many plants have been observed having yellowing and dwarfing, sometimes with leaf streaking and mosaic patterns. The extent of infection with viruses in collected samples varied from 0 to 100% in different fields. Reduction in cereal production caused by virus infections may be increased when vectors transmit the viruses early in the season. CYDV-RPV was determined in lower infection ratio that may be attributed to lack of effective transmissibility by the vector. WDV is reported as one of the major factor reducing cereal yields in Hungary, France, Slovakia and Sweden (Bendahmane et al. 1995; Lindsten et al. 1970; Pocsai et al. 2003; Vacke 1988). WDV was found in 17 out of the 26 tested fields. Yield losses in Muratli by WDV suggest that epidemics might be expected for many yr depending on the variety, the vector and the environmental conditions favoring symptom development. WDV seems a major threat for wheat as well as barley yellow dwarf viruses in Tekirdag.
The presence of BSMV indicated that pollen and seed transmission of cereal viruses may cause epidemics for many yr (Carroll 1980). BSMV is an economical virus disease because it reduces vigor and causes floret sterility (Tosic 1971). The yield losses depend on time of infection and susceptibility of the variety. Barley yields may be reduced by 60% (Sutic 1999). BSMV was found in three out of seven surveyed counties indicating the importance of preventing virus infection by pollen or seed transmission. The seed and pollen transmission is expected to increase the infection ratio for many yr if the same seed are used in the following yr. Wheat is natural host of the BMV. The virus can reduce plant growth and yields, number of tillering may be reduced by 50% in susceptible varieties (Tosic 1971). Because of mixed infections, it is very difficult to differentiate the characteristic symptoms in the field. BMV was founded widespread in Sarköy. Although we expected the virus widespread, no infection was found in three counties. BMV may also cause yield losses in cereals other than wheat.
Many wild monocotyledonous plants were found in wheat fields as wild oats (Avena spp.) and Johnson grass (Sorghumhalepense (L.) Pers.), Bromus spp., Secale cereale L. and Agropyron spp. which may serve as hosts and sources of surveyed six viruses.
As a result of the study, control of the viral infections in this area seems very difficult because of presence of efficient virus vectors for economically important luteoviruses and wild hosts of the viruses. More efforts and studies are needed to determine the epidemiological status of the viruses and to assess the extent of the presence of specialized viruses in wheat grown in Turkey.
Appendices
References
- Anonymous. 2001. Tekirdag Tarim Il Müdürlügü 2001 Yili Tarim Raporu. Tarim ve Köyigleri Bakanligi. Ocak 2002. s.8. [Tekirdag Provincial Agricultural Directory 2001, Annual Agricultural Report January 2001, in Turkish].
- Anonymous. 2003. Agriculture production. FAOSTAT Agriculture data. http://www.fao.org/waicent/portal/statistics_en.asp
- Atabekov, J.G., and V.K. Novikov. 1989. Barley stripe mosaic virus. Descriptions of plant viruses, No. 344. C.M.I./A.A.B., Kew, Surrey, England.
- Bendahmane, M., F. Jouanneau, F. de Kouchkovsky, H. Lapierre, I. Lebrun, and B. Gronenborn. 1995. Identification and characterization of wheat dwarf geminivirus from France. Agronomie 15(7-8) : 498.
- Bennett, C.W. 1969. Seed transmission of plant viruses. Adv. Virus Res. 14 : 221-261.
- Bisztray, G., and R. Gaborjanyi. 1989. Isolation and characterization of wheat dwarf virus found for the first time in Hungary. Z. Pflanzenkr. Pflanzen. 96 : 449-454.
- Bremer, K. 1971. Wheat streak mosaic virus in Turkey. Phytopathol. Mediterr. 10 : 280-282.
- Bremer, K., and M. Raatikainen. 1975. Cereal diseases transmitted or caused by aphids and leafhoppers in Turkey. Ann. Acad. Sci. Fenn. A, IV (Biologica) 203 : 1-14.
- Carroll, T.W. 1980. Barley stripe mosaic virus: its economic importance and control in Montana. Plant Dis. 64 : 136-140.
- Conti, M., C.J. D’Arcy, H. Jedlinski, and P.A. Burnett. 1990. The “yellow plage” of cereals, barley yellow dwarf virus. Pages 1-6 in P.A. Burnett (ed.), World perspectives on barley yellow dwarf. CIMMYT, Mexico DF, Mexico.
- Cooper, J.I. 1988. Brome mosaic virus (BMV). Page 69 in I.M. Smith, J. Dunez, D.H. Phillips, R.A. Lelliott, and S.A. Archer, (eds.), European handbook of plant diseases. Blackwell Scientific Publications, Oxford.
- El-Yamani, M., and J.H. Hill. 1990. Identification and importance of barley yellow dwarf virus in Morocco. Plant Dis. 74 : 291-294.
- Fitzgerald, P.J., and W.N. Stoner. 1967. Barley yellow dwarf studies in wheat (Triticum aestivum L.) I. Yield and quality of hard red winter wheat infected with barley yellow dwarf virus. Crop Sci. 7 : 337-341.
- Gill, C.C. 1969. Annual variation in strains of barley yellow dwarf virus in Manitoba and the occurrence of green bug-spesific isolates. Can. J. Bot. 47 : 1277-1283.
- Hammond, J., R.M. Lister, and J.E. Foster. 1983. Purification, identity and some properties of an isolate of barley yellow dwarf virus by enzyme-linked immunosorbent assay. J. Gen. Virol. 69 : 649-654.
- Harrison, B.D. 1999. Steps in the development of Luteovirology. Pages 1-14 in H.G. Smith and H. Barker (eds.), The Luteoviridae. Oxford University Press, USA.
- Hofmann, T.K., and F.L. Kolb. 1998. Effects of barley yellow dwarf virus on yield and yield components of drilled winter wheat. Plant Dis. 82 : 620-624.
- Köse, A., and F. Ertunç. 1999. Virus diseases of wheat and barley in Eskisehir province. J. Turkish Phytopathol. 28 (1-2) : 55-62.
- Kurçman, S. 1981. Eskisehir ili’nde bugdaylarda görülen bugday mosaic virüs hastaligi üzerine arastirmalar. Bitki Koruma Bülteni, 21(1) : 1-17. [Research on wheat mosaic virus disease on wheat in Eskisehir province, in Turkey].
- Lane, L.C. 1977. Brome mosaic virus. Description of plant viruses No. 183. C.M.I./A.A.B., Kew, Surrey, England.
- Lindsten, K., J. Vacke, and B. Gerhardsen. 1970. A preliminary report on three cereals virus diseases new to Sweden spread by Macrosteles- and Psammottettix-leafhoppers. Natl. Swed. Inst. Plant Prot. Contribution 14 : 283-297.
- Lister, R.M., and W.F. Rochow. 1979. Detection of barley yellow dwarf virus by enzyme-linked immunosorbent assay. Phytopathology 69 : 649-654.
- Makkouk, K.M., O.I. Azzam, J.S. Skaf, M. El-Yamani, C. Cherif, and A. Zouba. 1990. Situation review of barley yellow dwarf virus in the West Asia and North Africa. Pages 61-65 in P.A. Burnett (ed.), World perspectives on barley yellow dwarf. CIMMYT, Mexico DF, Mexico.
- Makkouk, K.M., D.E. Leseman, E.E. Saasi, F. Altay, B. Süzen, N. Bolat, H.J. Braun, T.S. Payne, and S.P.S. Bennival. 1994. Identity of and screening for resistance to a new soil-borne virus affecting wheat in Turkey. 9th Congr. Phytopathol. Union, Kusadasi-Aydin, Türkey, 18-24 September 1994, pp. 271-272.
- Mayo, M.A., and C.J. D’Arcy. 1999. Family Luteoviridae: A reclassification of Luteoviruses. Pages 15-22 in H.G. Smith and H. Barker (eds.), The Luteoviridae. Oxford University Press, USA.
- McGrath, P.F., and J.S. Bale. 1990. The effects of sowing dates and choice of insecticide on cereal aphids and barley yellow dwarf virus epidemiology in Northern England. Ann. Appl. Biol. 117 : 31-43.
- McKirdy, S.J., and R.A.C. Jones. 1997. Effect of sowing time on barley yellow dwarf virus infection in wheat : Virus incidence and grain yield losses. Aust. J. Agric. Res. 48 : 199-206.
- McKirdy, S.J., R.A.C Jones, and F.W. Nutter Jr. 2002. Quantification of yield losses caused by barley yellow dwarf virus in wheat and oats. Plant Dis. 86 : 769-773.
- McNeal, F.H., M.A. Berg, and T.W. Carroll. 1976. Barley stripe mosaic virus data from six infected spring wheat cultivars. Plant Dis. Rep. 60 : 730-733.
- Miller, W.A., S. Liu, and R. Beckett. 2002. Pathogen profile: Barley yellow dwarf virus: Luteoviridae or Tombusviridae? Mol. Plant Pathol. 3(4) : 177-183.
- Najar, A., K.M. Makkouk, H. Boudhir, S.G. Kumari, R. Zarouk, R. Bessai, and F.B. Othman. 2000. Viral diseases of cultivated legume and cereal crops in Tunisia. Phytopathol. Mediterr. 39(3) : 423-432.
- Oswald, J.W., and B.R. Houston. 1951. A new virus disease of cereals transmissible by aphids. Plant Dis. Rep. 35 : 471-475.
- Plumb, R.T. 1983. Barley yellow dwarf virus-a global pro-blem. Pages 185-198 in R.T. Plumb and J.M. Thresh (eds.), Plant virus epidemiology-The spread and control of insect-borne viruses. Blackwell Scientific Publications, Oxford.
- Pocsai, E., A. Çitir, G. Köklü, I. Murányi, K. Nyerges, G. Vida, és R. Zsovákné Hangyál. 2003. Levélsárgulás és törpeség tünetet okozó gabonavírusok Törökországban. XIII. Keszthelyi Növényvédelmi Fórum 2003. Keszthely. 2003. január 29-31. pp. 37-39. [Leaf yellowing and dwarfing caused by cereal viruses in Turkey, in Hungarian].
- Pringle, C.R. 1999. Virus taxonomy-1999. Arch. Virol. 144 : 421-429.
- Rochow, W.F. 1969. Biological properties of four isolates of barley yellow dwarf virus. Phytopathology 59 : 1580-1589.
- Rochow, W.F. 1979. Field variants of barley yellow dwarf virus: Detection and fluctuation during twenty years. Phytopathology 69 : 655-660.
- Sutic, D.D. 1999. Virus diseases of food graminoid plants. Pages 1-71 in D.D. Sutic, R.E. Ford and M.T. Tosic (eds.), Handbook of plant virus diseases. CRC Press, Boca Raton, Fla, USA.
- Tosic, M. 1971. Virus diseases of wheat in Serbia. I. Isolation and determination of wheat streak mosaic virus and brome mosaic virus. Phytopathol. Z. 70 : 145-162.
- Vacke, J. 1961. Wheat dwarf virus disease. Biol. Plant. (Praha) 3 : 228-233
- Vacke, J. 1988. Occurrence and economical importance of wheat dwarf virus in Czeshoslovakia. 5th Conf. on Virus Diseases of Gramineae in Europe. Budapest, 24-27 May 1988. p. 43.
List of figures
Figure 1
Location of collected wheat samples in Tekirdag province, Turkey.
Figure 2
Infection ratio (%) of tested viruses in wheat samples from seven counties in Tekirdag, Turkey in 2003.
List of tables
Table 1
Number of virus infected wheat fields in Tekirdag, Turkey in 2003
Table 2
Number of virus infected plants in tested wheat samples in Tekirdag, Turkey in 2003
Table 3
Infection types of cereal viruses in tested wheat samples in seven counties in Tekirdag, Turkey in 2003