Abstracts
Abstract
Pythium species cause seed rot (SR) and damping-off (DO) in soybean worldwide. In a previous study, a number of Pythium species were isolated from infected soybean plants across Ontario and Quebec, but their comparative pathogenicities to soybean were not examined. In the present research, 24 isolates from eight Pythium spp. were evaluated for their pathogenicity in causing soybean SR and DO in a greenhouse environment. The effect of temperature on the ability of these isolates to cause SR was also studied. There were significant differences among the eight Pythium spp. for both SR and DO. When tested at 25°C, Pythium ultimum was the most pathogenic species, causing 97.0% SR and 46.4% DO, on average, in the two soybean cultivars used. Pythium aphanidermatum was the second most pathogenic species, resulting in 88.5% SR and 41.8% DO. The two species resulted in significantly greater SR and DO than the other six species tested and were considered highly pathogenic. Of the two cultivars used in these trials, ‘Beechwood’ was significantly more susceptible than ‘Nattawa’ to both SR and DO. Temperature had a significant influence on SR caused by Pythium spp. At all four temperatures tested (4°C, 12°C, 20°C and 28°C), P. ultimum was highly pathogenic, while P. arrenomanes, P. coloratum and P. dissotocum were the least pathogenic. The interactions between temperature and Pythium spp. were more pronounced for P. aphanidermatum, which showed an increased percentage of SR with an increase in temperature, and for P. irregulare, P. macrosporum and P. sylvaticum, which showed a decreased percentage of SR with an increase in temperature.
Keywords:
- damping-off,
- Glycine max,
- pathogenicity,
- Pythium spp.,
- seed rot,
- soybean
Résumé
Les espèces de Pythium provoquent la pourriture de racine (PR) et la fonte des semis (FS) chez la fève de soja dans le monde entier. Dans une étude précédente, des espèces de Pythium ont été isolées à partir de plants de fève de soja infectés en Ontario et au Québec, mais leur pouvoir pathogène n’a pas été évalué. Dans la présente recherche, le pouvoir pathogène de 24 isolats de huit espèces de Pythium a été évalué relativement à leur capacité de provoquer la PR et la FS dans des serres; l’effet de la température sur leur capacité de provoquer la PR a également été étudié. Il y avait des différences significatives entre les huit espèces de Pythium pour la PR et la FS. À 25°C, P. ultimum détenait le plus grand pouvoir pathogène, provoquant 97,0 % de PR et 46,4 % de FS, en moyenne, chez les deux cultivars utilisés. Pythium aphanidermatum détenait le deuxième plus grand pouvoir pathogène, provoquant 88,5 % de PR et 41,8 % de FS. Des deux cultivars utilisés dans ces essais, ‘Beechwood’ était significativement plus susceptible que ‘Nattawa’ à la PR et à la FS. La température a eu un effet significatif sur la PR. Pour les quatre températures évaluées (4°C, 12°C, 20°C et 28°C), P. ultimum détenait un important pouvoir pathogène, alors que P. arrenomanes, P. coloratum et P. dissotocum étaient les moins pathogènes. L’influence de la température était plus prononcée chez P. aphanidermatum, qui montrait un pourcentage élevé de PR avec une augmentation de la température, et chez P. irregulare, P. macrosporum et P. sylvaticum, qui ont montré une diminution de PR avec une augmentation de la température.
Mots clés:
- fève de soja,
- fonte des semis,
- Glycine max,
- pourriture de racine,
- pouvoir pathogène,
- Pythium spp
Article body
Introduction
Pythium spp. are capable of causing plant diseases individually, but several species are frequently isola-ted from a single plant (Dorrance et al. 2004). Typical symptoms of infection by Pythium spp. include soft and decayed seed before germination, pre- or post-emergence damping-off in the seeding stage, and hypocotyl discoloration and root rot in advanced growth stages (Rosso et al. 2008; Yang 1999).
Pythium root rot of soybean, commonly referred to as Pythium complex, is found in all soybean- producing regions of the world (Yang 1999). Pythium complex is a serious problem for soybean seedling establishment in the USA, and disease severity increases with cool and moist conditions, minimum tillage and earlier planting (Broders et al. 2007).
Previous studies have shown that a number of Pythium spp. might be pathogenic to soybean (Brown and Kennedy 1965; Thomson et al. 1971; Zhang and Yang 2000). Van der Plaats-Niterink (1981) reported that Pythium species, including P. aphanidermatum (Edson) Fitzp., P. irregulare Buisman, P. oligandrum Drechsler, P. ultimum Trow, P. vexans de Bary, and group HS (hyphal swellings), were associated with soybean roots. Bates et al. (2008) demonstrated that these species were pathogenic in soybean. In addition, Kirkpatrick et al. (2006a) reported that 47% of 208 selected isolates of Pythium spp. were pathogenic to soybean and were moderately to highly aggressive, based on plant emergence and root discoloration. Zhang and Yang (2000) showed that the population of Pythium spp. collected from corn- soybean rotation fields contained high frequencies of isolates pathogenic to both crops.
There is no information on the different levels of aggressiveness among isolates within a Pythium sp. to soybean. Studies in other crops have shown that isolates within a Pythium sp. can vary in aggressiveness (Chagnon and Bélanger 1991; Hendrix and Campbell 1973; McCarter and Littrell 1970; Zhang and Yang 2000). Moorman and Kim (2004) demonstrated that several P. irregulare isolates were highly pathogenic to geranium (Pelargonium × hortorum), while others were relatively weakly pathogenic.
Temperature is another important factor affecting the pathogenicity of Pythium spp. Pythium debaryanum Auct. Non R. Hesse and P. ultimum greatly reduced soybean seed germination below 24°C (Thomson et al. 1971). Pythium aphanidermatum reduced seed germination only above 20°C (Ben-Yephet and Nelson 1999; Thomson et al. 1971), and P. irregulare caused cucumber damping-off only from 20 to 24°C (Ben-Yephet and Nelson, 1999). Abad et al. (1994) reported that isolates of P. volutum Vanterp. & Truscott from North Carolina were more aggressive on turf grass from 28 to 32°C than at 16°C, while Feng and Dernoeden (1999) reported that P. volutum isolates from Maryland were more aggressive on bentgrass at 18°C than at 28°C.
The method commonly used for assessing the pathogenicity of Pythium spp. in causing seed rot involves placing seeds directly on a Pythium culture growing in a Petri dish (Broders et al. 2007; Brown and Kennedy 1965; Dorrance et al. 2004; Thomson et al. 1971; Zhang and Yang 2000). Pre-emergence damping-off is commonly assessed using a pot assay, in which inoculum is mixed with soil or another grow-ing medium and seeds are subsequently planted in the medium (Ali-Shtayeh et al. 2003; Bates et al. 2008; Broders et al. 2007; Kirkpatrick et al. 2006a, 2006b; Zhang and Yang 2000). Alternatively, a plug of Pythium isolate can be placed directly on the hypocotyl to induce infection (Rosso et al. 2008; Thomson et al. 1971).
The objectives of this study were to compare the pathogenicity of 24 isolates from eight Pythium spp. in causing seed rot (SR) and damping-off (DO) in soybean and to determine the influence of temperature on SR.
Materials and methods
Pythium isolates and soybean cultivars
Twenty-four isolates from eight Pythium spp., inclu-ding P. aphanidermatum (3 isolates), P. arrenomanes Drechs. (4), P. coloratum Vaartaja (2), P. dissotocum Drechs. (2), P. irregulare (4), P. macrosporum Vaartaja & Plaäts-nit. (2), P. sylvaticum W.A. Campbell & J.W. Hendrix (2), and P. ultimum (5), were obtained from the Canadian Collection of Fungal Cultures (CCFC) located at the Eastern Cereal and Oilseed Research Centre (ECORC) of Agriculture and Agri-Food Canada (AAFC) and used in the study. These Pythium isolates were either recovered from the soil or unknown species of field and horticultural crops from different geographic regions of British Columbia (13), Alberta (2), Manitoba (2), Ontario (3) and Quebec (4) from 1973 to 2002. The isolates had been preserved in liquid nitrogen since their deposition at the CCFC and were cultured at 28°C on V8 agar (100 mL V8 juice, 0.6 g CaCO3, and 20 g agar L‑1) both in slants and Petri dishes for use in the present study. Pure cultures of these isolates were confirmed by internal transcribed spacer (ITS) sequencing for species identity at the CCFC Research Laboratory. Cultures were maintained on V8 agar at 4°C and transferred every 3 mo for a maximum of three transfers during the course of this study.
Soybean cultivars Beechwood and Nattawa were used in the experiments to evaluate the comparative pathogenicity of the eight Pythium spp., and cultivar PS50 was used to determine the influence of tempe-rature on their pathogenicity. ‘Beechwood’ and ‘PS50’ are considered susceptible and ‘Nattawa’ is mode-rately resistant to root rot under field conditions (E.R. Cober, AAFC, pers. comm.). Seeds of these soybean cultivars were provided by the AAFC soybean breeding program.
Seed rot (SR) tests
A 5-mm2 V8 agar plug of Pythium isolate was placed at the centre of a 9-cm Petri dish containing 20 mL water agar. Petri dishes were kept at 22°C for 3 d, then 10 seeds of soybean cultivar were added to each plate. Seeds were spaced equally, approximately 2-cm apart, in each Petri dish. The seeds were previously surface sterilized in 0.25% sodium hypochlorite solution (The Clorox Company, Oakland, CA, USA) for 1 min, then rinsed in sterile distilled water. The Petri dishes were incubated at 25°C with a 12 h photoperiod at a light intensity of 250 mol.m‑2.s‑1 for 7 d, and the number of rotted seeds per plate was recorded. Four plates that were inoculated with ste-rile V8 agar plugs for each of the two soybean cultivars were included as controls for the existence of possible extraneous airborne or seedborne inoculum. SR was calculated based on four replicate Petri dishes for each isolate by cultivar combination in each experiment. The experiment was arranged in a two-factor (species and cultivar) nested design, with isolates nested within species and repeated once.
Damping-off (DO) tests
Six 1-cm2 plugs of V8 agar with 3-d-old cultures of each Pythium isolate were placed in 500 mL flasks containing 200 mL of sand (U.S. Silica Company, Berkeley Springs, WV, USA), 11.2 mL of corn meal, and 80 mL of deionized water that had been autoclaved for 40 min and then autoclaved again 24 h later to prepare a large quantity of inoculum for greenhouse inoculation. The isolates were allowed to colonize the sand-cornmeal medium at room temperature for 9 d prior to being used for inoculation. The flasks were shaken every other day to ensure uniform colonization.
The soybean seeds were surface sterilized as previously described, then soaked in sterile distilled water for approximately 6 h. Seeds were kept moist and at room temperature for 2 d until germination.
Plants were grown in planting trays (58 cm × 28 cm × 8 cm) consisting of 72 cells (4.5 cm × 4.5 cm × 5 cm). The cells were each filled with a base layer of 30 g of Pro-mix soil (Plant Products Ltd., Brampton, ON, Canada), followed by a layer of 2.5 g of Pythium inoculum. Two germinated soybean seeds per cell were planted directly on the inoculum and covered with an additional 2 g of Pro-mix soil. Four replicate planting trays for each isolate by cultivar combination were used in each experiment. The trays were placed in the greenhouse with a 16-h photoperiod and temperature of 24°C during the day and 18°C at night. Trays were watered once per day to maintain soil moisture. The number of seedlings that emerged and survived after emergence was recorded 10 d after planting.
Four planting trays that were inoculated with sterile sand-cornmeal medium for each of the two soybean cultivars were included as controls for the presence of possible extraneous soilborne inoculum. The calculation of the percentage of DO and the experimental design were the same as the ones described earlier for SR tests, and the experiment was repeated once.
Effect of temperature
The effect of temperature on the pathogenicity of the eight Pythium spp. in causing SR was examined at 4°C, 12°C, 20°C and 28°C in growth cabinets. The PS50 soybean seed used in this experiment was surface sterilized as described earlier for the SR tests. Seed placement and seed rot assessment method also were the same. For each isolate and temperature combination, four replicate Petri dishes were assessed for SR 7 d after plating in each experiment. Petri dishes were arranged in a completely rando-mized design in each growth cabinet, and the experiment was repeated once.
Statistical analyses
Residuals for each parameter for each experiment were examined for normality and homogeneity of variances. An angular transformation of percent reductions in SR and DO was used in the analysis of variance to stabilize variances (Snedecor and Cochran 1980). Treatment means of the untransformed data were presented and separated by Fisher’s least significant difference (LSD) test at a probability level of P ≤ 0.05, based on the analyses of transformed data. The assumption of normality based on Shapiro Wilk’s test was examined using the Univariate Procedure of SAS and the random and homogeneous distribution of residuals was assessed using the Plot Procedure (SAS Institute Inc. 2004). Data from the repeated experiments were analyzed separately and in a combined analysis using SAS/STAT® mixed models (Littell et al. 1996) with experiments and interactions with experiments considered as random effects. Heterogeneity of variances among experiments was checked for each parameter with a likelihood ratio test (LRT) comparing the diffe-rence of -2 log likelihood of a homogeneous and he-terogeneous variance model with the c2 distribution with 2 degrees of freedom. The significance of interactions with experiments were also examined with LRT by comparing models with and without selected interactions (Wolfinger 1993). Non-significant interactions were pooled with error. Contrasts were used to compare the two isolates within each species of P. coloratum, P. dissotocum, P. macrosporum and P. sylvaticumi, and significance is at the probability level P ≤ 0.05 unless otherwise indicated, based on the analyses of transformed data.
Results
Pathogenicity of Pythium spp.
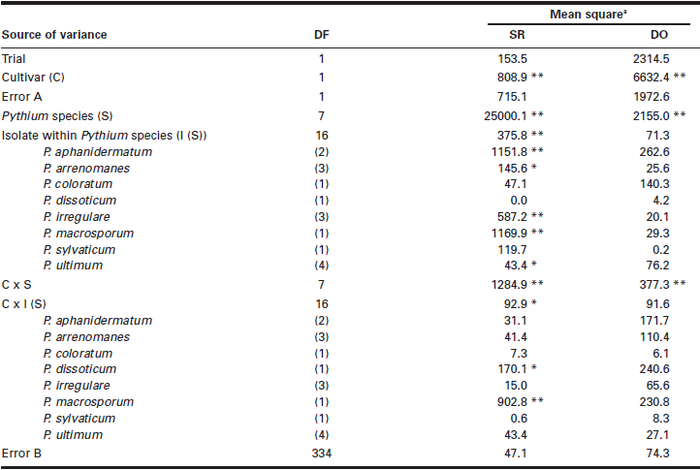
Significant differences (P < 0.01) were observed in both SR and DO among the eight Pythium spp. between the two soybean cultivars and in Pythium spp. x cultivar interactions (Table 1). The effects of Pythium spp. x cultivar interactions, although statistically significant, were relatively small and represented less than 5% of the total treatment effects for both SR and DO. As a result, the pathogenicity of Pythium spp. was calculated based on each soybean cultivar and the mean of the two cultivars, and vice versa for the differences in susceptibility to Pythium spp. between the two cultivars. Significant differences among isolates within species were observed in SR for P. aphanidermatum, P. arrenomanes, P. irregulare and P. macrosporum, and significant cultivar x isolate interactions were observed for P. dissoticum and P. macrosporum.
The eight Pythium spp. showed different levels of pathogenicity to soybean, with SR ranging from 28.1 to 95.3% in ‘Beachwood’ and from 20.8 to 98.8% in ‘Nattawa’, and DO ranging from 25.4 to 58.1% in ‘Beachwood’ and from 18.5 to 34.6% in ‘Nattawa’ (Table 2). On average for the two cultivars, P. ultimum had the greatest SR (97.0%) and DO (46.4%), followed by P. aphanidermatum, which caused 88.5% SR and 41.8% DO. These two species resulted in significantly greater SR and DO than the other six species tested and were considered highly pathogenic. The remaining species resulted in low levels of SR (24.5 to 49.2%) and DO (22 to 26.3%) and were considered weakly pathogenic, even though there were significant differences among these species for SR.
Table 1
Mean squares from the analysis of variance for the effect of Pythium species, isolates within species, soyban cultivar, and their interactions in seed rot (SR) and damping-off (DO)
Seed rot and damping-off expressed in percentage were angular-transformed before the analysis of variance was performed;
** = P < 0.01;
* = P < 0.05;
no asterisk = P > 0.05.
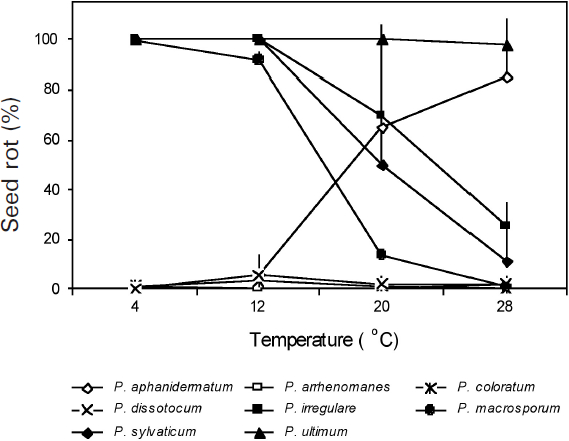
Figure 1
Quantitative differences in percentage of soybean seed rot caused by eight Pythium spp. as affected by temperature 7 d after inoculation
The percentage of seed rot for each species is the mean of two to five isolates and 40 seeds per isolate in each of the two experiments. Vertical bars represent standard deviations.
Table 2
Variations among isolates and species of eight Pythium spp. causing seed rot (SR) and damping-off (DO) in two soybean cultivarsa
Data are means of two trials. SR and DO expressed in percentage were angular-transformed before the analysis of variance was performed.
Means followed by the same letter in a column among isolates under each Pythium species, among Pythium spp. under species average, or among soybean cultivars under cultivar average were not significantly different at P = 0.05.
Accession numbers for isolates maintained in the Canadian Collection of Fugal Cultures.
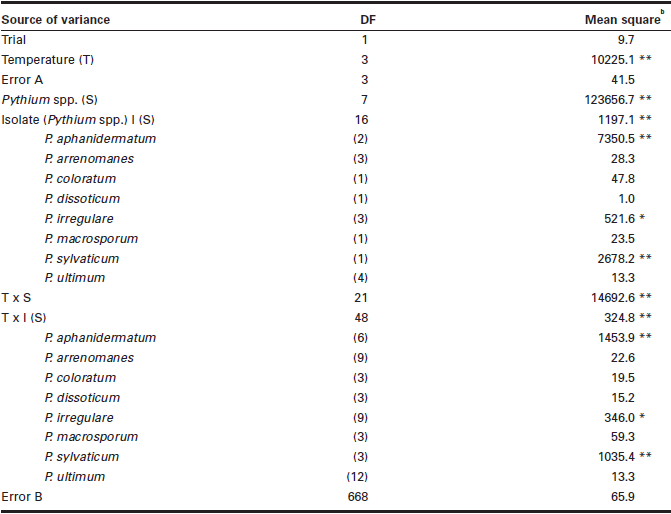
Table 3
Mean squares from analysis of variance for the effect of temperature on the pathogenicity of eight Pythium spp. in causing seed rot of soybeana
Seed rot data expressed in percentage were angular-transformed before the analysis of variance was performed.
** = P < 0.01; * = P < 0.05; no asterisk P > 0.05.
Both the Beechwood and Nattawa cultivars were susceptible, but the former showed more severe SR (54.1%) and DO (34.5%) than the latter, which had 44.0% SR and 23.4% DO when averaged over the eight Pythium spp. (Table 2).
Effect of temperature
There were significant differences among temperatures, Pythium spp., and the temperature × Pythium spp. interaction in SR (Table 3). Significant differences were also observed among isolates within species and temperature × isolate interactions for P. aphanidermatum,P. irregulare and P. sylvaticum. At each of the four temperatures tested, all isolates of P. ultimum were highly pathogenic, causing > 96% SR, while isolates of P. arrenomanes, P. coloratum and P. dissotocum were weakly pathogenic, causing < 7% SR (Fig. 1). The remaining four Pythium spp. caused different degrees of SR depending on the temperature. With the increase in temperature from 4°C to 28°C, the percentage of SR caused by P. aphanidermatum increased while that of P. irregulare,P. macrosporum and P. sylvaticum decreased. Pythium aphanidermatum caused < 1% SR at 4°C and 5.8% SR at 12°C, but 65.4% SR at 20°C and 85.4% SR at 28°C. In contrast, P. irregulare,P. macrosporum and P. sylvaticum each caused > 95% SR at 4°C and 12°C, 18.2 to 69.7% SR at 20°C, and only 8.2 to 25.0% SR at 28°C.
Discussion
Of the eight Pythium spp. evaluated, Pythium aphanidermatum, P. irregulare, P. sylvaticum and P. ultimum had previously been reported to be pathogenic to soybean (Bates et al. 2008; Rizvi and Yang 1996; Thomson et al. 1971; Van der Plaats-Niterink 1981; Yang 1999). However, no studies had examined the comparative pathogenicity of these Pythium spp. in causing SR and DO in soybean. The present research demonstra-ted that only P. aphanidermatum and P. ultimum were highly pathogenic, causing > 88% SR and > 40% DO, while the other six species tested were weakly pathogenic at 25°C (Table 2). In addition, this study demonstrated that P. aphanidermatum, P. irregulare, P. macrosporum and P. sylvaticum are temperature dependent in causing soybean SR. Of these temperature-dependent pathogenic species, only P. aphanidermatum and P. irregulare had previously been recognized as pathogens of soybean (Ben-Yephet and Nelson 1999; Thompson et al. 1971). Pythium aphanidermatum, although highly pathogenic at 25°C, showed little or no pathogenicity at 4°C and 12°C (Fig. 1). These results suggest that P. aphanidermatum is probably not responsible for causing soybean root rot and damping-off in regions with short soybean growing seasons where soil temperatures often are below 20°C during crop emergence and the early seedling development stage. In contrast, P. macrosporum, P. irregulare and P. sylvaticum, which were weakly pathogenic at 25°C, were highly pathogenic at low temperatures, causing > 90% SR at both 4°C and 12°C (Fig. 1). These species may have a greater impact on short-season soybean production than P. aphanidermatum. However, the effect of temperature on P. irregulare observed in the present study is somewhat different from that reported by Ben-Yephet and Nelson (1999), who found that P. irregulare caused cucumber seedling damping-off only at 20°C and 24°C. It is possible that different isolates of P. irregulare can have different optimal temperatures for pathogenicity. In addition, P. irregulare is known for its variable morphological and genetic characters, and several distinct groups and a new species (P. crypto-irregulare) within the P. irregulare complex have been reported in recent taxonomic studies (Garzon et al. 2005, 2007; Matsumoto et al. 2000). It is also possible that the P. irregulare isolates used by Ben-Yephet and Nelson (1999) came from more than one species.
The high level of pathogenicity of P. macrosporum to soybean at low temperatures had not been reported previously. Pythium macrosporum has been isolated in several countries, including Canada, Germany, Japan, the Netherlands and the United States (Allain-Boule et al. 2004; Uzuhashi et al. 2008; Van der Plaäts-Niterink 1981; Van Os et al. 1999; Westover and Bever 2001), and is known to cause root rots in flower bulbs (Westover and Bever 2001), grasses (Van Os et al. 1999), and carrot (Allain-Boule et al. 2004). This species was detected in diseased soybean roots using a Pythium DNA array hybridization method during an extensive survey for root rot pathogens in commercial fields of soybean in eastern Ontario and Quebec (Barasubiye et al. 2005). The high levels of pathogenicity of the two P. macrosporum isolates to soybean observed in this research suggest that soybean could be a potential host for P. macrosporum, which has been identified as a pathogen in other plant species. This species could have a significant negative impact on soybean stands in eastern Ontario, Quebec and southern Manitoba, where most of the Canadian short-season soybean is grown, and where soil temperatures are below 20°C during crop emergence and early stages of plant growth. Further studies including a large number of P. macrosporum isolates from soybean and various host plants are needed to better understand the effect of temperature on P. macrosporum isolates × soybean cultivar interactions.
There were significant differences among isolates within P. aphanidermatum, P. arrenomanes, P. irregulare and P. macrosporum in causing SR (Tables 2 and 3). These results are in agreement with those of Martin and Loper (1999) who found that the pathogenicity responses of Pythium species can be isolate -specific. The presence of different levels of aggressi-veness among isolates within the pathogenic Pythium spp. has practical implications that must be considered when screening and breeding soybean for Pythium root rot resistance. It is important that aggressive isolates be used because isolates with low aggressiveness may not be able to discriminate between lines with different levels of resistance; perhaps a mixture of several different isolates should be used to screen for resistance.
Soybean cultivar resistance to Pythium spp. has recently been identified (Bates et al. 2008), making resistance breeding possible and a viable strategy for managing Pythium SR and DO. Of the two soybean cultivars used in the pathogenicity experiment in the present study, Nattawa was significantly more resis-tant than Beechwood (Table 2). The cultivars’ reactions were in agreement with previous field observations. Although the cultivar × Pythium spp. interactions were significant for both SR and DO (Table 1), the differential responses of the two cultivars to the highly pathogenic species were less apparent (Table 2). These results indicate that soybean may share common genes for resistance with these pathogenic species and that breeding for resis-tance to one Pythium species may also give enhanced resistance to other Pythium spp. Further research is needed to confirm the presence and heritability of resistance genes in ‘Nattawa’ and their usefulness in future cultivar development.
Appendices
Acknowledgements
This research was funded by the Ontario Soybean Growers. We thank Drs. A. Lévesque and T. Barasubiye for advice and suggestions on the choice of Pythium spp., and A. Nagasawa and Y. Chen for technical assistance and statistical analyses.
References
- Abad, Z.G., H.D. Shew, and L.T. Lucas. 1994. Characterization and pathogenicity of Pythium species isolated from turf grass with symptoms of root and crown rot in North Carolina. Phytopathology 84 : 913-921.
- Ali-Shtayeh, M., A.M.A. Salah, and R.M. Jamous. 2003. Ecology of hymexazol-insensitive Pythium species in field soils. Mycopathologia 156 : 333-342.
- Allain-Boule, N., C.A. Lévesque, C. Martinet, R.R. Bélanger, and R.J. Tweddell. 2004. Identification of Pythium species associated with cavity spot lesions on carrots in eastern Quebec. Can. J. Plant Pathol. 26 : 365-370.
- Barasubiye, T., K.A. Seifert, A.U. Tenuta, S. Rioux, T.R. Anderson, and C.A. Lévesque. 2005. Application of DNA methods to identify and detect Pythium, Phytophthora, and Fusarium spp. associated with soybean root rot in eastern Ontario and Québec. Can. J. Plant Pathol. 27 : 464-465.
- Bates, G.D., C.S. Rothrock, and J.C. Rupe. 2008. Resistance of the soybean cultivar Archer to Pythium damping-off and root rot caused by several Pythium spp. Plant Dis. 92 : 763-766.
- Ben-Yephet, Y., and E.B. Nelson. 1999. Differential suppression of damping-off caused by Pythium aphanidermatum, P. irregulare, and P. myiotylum in composts at different temperatures. Plant Dis. 83 : 356-360.
- Broders, K.D., P.E. Lipps, P.A. Paul, and A.E. Dorrance. 2007. Characterization of Pythium spp. associated with corn and soybean seed and seedling disease in Ohio. Plant Dis. 91 : 727-735.
- Brown, G.E., and B.W. Kennedy. 1965.Pythium pre-emergence damping-off of soybean in Minnesota. Plant Dis. Rep. 49 : 646.
- Chagnon, M.C., and R.R. Bélanger. 1991. Tolerance in greenhouse geraniums to Pythium ultimum. Plant Dis. 75 : 820-823.
- Dorrance, A.E., S.A. Berry, P. Browen, and P.E. Lipps. 2004. Characterization of Pythium spp. from three Ohio fields for pathogenicity on corn and soybean and metalaxyl sensitivity. Plant Health Progress (online) doi: 10.1094/PHP-2004-0202-01-RS.
- Feng, Y., and P.H. Dernoeden. 1999.Pythium species associated with root dysfunction of creeping bentgrass in Maryland. Plant Dis. 83 : 516-520.
- Garzón, C.D., D.M. Geiser, and G.W. Moorman. 2005. Amplified fragment length polymorphism analysis and internal transcribed spacer and cox II sequences reveal a species boundary within Pythium irregulare. Phytopathology 95 : 1489-1498.
- Garzón, C.D., J.M. Yánez, and G.W. Moorman. 2007.Pythium cryptoirregulare, a new species within the P. irregulare complex. Mycologia 99 : 291-301.
- Hendrix, F.F., and W.A. Campbell. 1973.Pythium as plant pathogens. Annu. Rev. Phytopathol. 11 : 78-98.
- Kirkpatrick, M.T., J.C. Rupe, and C.S. Rothrock. 2006a. Soybean response to flooded soil conditions and the association with soilborne plant pathogenic genera. Plant Dis. 90 : 592-596.
- Kirkpatrick, M.T., C.S. Rothrock, J.C. Rupe, and E.E. Gbur. 2006b. The effect of Pythium ultimum and soil flooding on two soybean cultivars. Plant Dis. 90 : 597-602.
- Littell, R.C., G.A. Milliken, W.W. Stroup, and R.D. Wolfinger. 1996. SAS System for Mixed Models. SAS Institute Inc., Cary, NC, USA.
- Martin, F.N., and J.E. Loper. 1999. Soilborne plant disease caused by Pythium spp.: ecology, epidemiology and prospects for biological control. Plant Sci. 18 : 111-181.
- Matsumoto, C., K. Kageyama, H. Suga, and M. Hyakumachi. 2000. Intraspecific DNA polymorphisms of Pythium irregulare. Mycol. Res. 104 : 1333-1341.
- McCarter, S.M., and R.H. Littrell. 1970. Comparative pathogenicity of Pythium aphanidermatum and Pythium myriotylum to twelve plant species and intraspecific variation in virulence. Phytopathology 60 : 264-268.
- Moorman, G.W., and S.H. Kim. 2004. Species of Pythium from greenhouses in Pennsylvania exhibit resistance to propamocarb and mefenoxam. Plant Dis. 88 : 630-632.
- Rizvi, S.S.A., and X.B. Yang. 1996. Fungi associated with soybean seedling disease in Iowa. Plant Dis. 80 : 57-60.
- Rosso, M.L., J.C. Rupe, P. Chen, and L.A. Mozzoni. 2008. Inheritance and genetic mapping of resistance to Pythium damping-off caused by Pythium aphanidermatum in ‘Archer’ soybean. Crop Sci. 48 : 2215-2222.
- SAS Institute Inc. 2004. SAS/STAT® Users’ Guide. Cary, NC, USA. 5121 p.
- Snedecor, G.W., and W.G. Cochran. 1980. Statistical Methods, 8th ed. The Iowa State University Press, Ames, IA, USA. 503 p.
- Thomson, T.B., K.L. Athow, and F.A. Laviolette. 1971. The effect of temperature on the pathogenicity of Pythium aphanidermatum, P. debaryanum, and P. ultimum on soybean. Phytopathology 61 : 933-935.
- Uzuhashi, S., M. Tojo, S. Kobayashi, K. Tokura, and M. Kakishima. 2008. First records of Pythiumaquatile and P. macrosporum isolated from soils in Japan. Mycoscience 49 : 276-279.
- Van der Plaäts-Niterink, A.J. 1981. Monograph of the genus Pythium. Stud. Mycol. 21 : 1-239.
- Van Os, G.J., J.P.M. Wijnker, and W.J.M. van Gulik. 1999. Effects of soil fumigation and flooding on suppression of Pythium root rot in ornamental bulb culture. Eur. J. Plant Pathol. 105 : 791-800.
- Westover, K.M., and J.D. Bever. 2001. Mechanisms of plant species coexistence: roles of rhizosphere bacteria and root fungal pathogens. Ecology 82 : 3285-3294.
- Wolfinger, R. 1993. Laplace’s approximation for nonlinear mixed models. Biometrika 80 : 791-795.
- Yang, X.B. 1999.Pythium damping-off and root rot. Pages 42-44 in G.L. Hartman et al. (ed.), Compendium of Soybean Diseases, 4th ed. APS Press, St. Paul, MN, USA.
- Zhang, B.Q., and X.B. Yang. 2000. Pathogenicity of Pythium populations from corn-soybean rotation fields. Plant Dis. 84 : 94-99.
List of figures
Figure 1
Quantitative differences in percentage of soybean seed rot caused by eight Pythium spp. as affected by temperature 7 d after inoculation
List of tables
Table 1
Mean squares from the analysis of variance for the effect of Pythium species, isolates within species, soyban cultivar, and their interactions in seed rot (SR) and damping-off (DO)
Table 2
Variations among isolates and species of eight Pythium spp. causing seed rot (SR) and damping-off (DO) in two soybean cultivarsa
Data are means of two trials. SR and DO expressed in percentage were angular-transformed before the analysis of variance was performed.
Means followed by the same letter in a column among isolates under each Pythium species, among Pythium spp. under species average, or among soybean cultivars under cultivar average were not significantly different at P = 0.05.
Accession numbers for isolates maintained in the Canadian Collection of Fugal Cultures.
Table 3
Mean squares from analysis of variance for the effect of temperature on the pathogenicity of eight Pythium spp. in causing seed rot of soybeana