Abstracts
Abstract
Carrot yield (Daucus carota) and population levels of the root-lesion nematode Pratylenchus penetrans and the northern root-knot nematode Meloidogyne hapla were measured in five rotation crops and in subsequent carrot crops at three field sites (1998-1999, 1999-2000, and 2000-2001). Total and marketable carrot yields averaged over the three sites did not differ in the crop sequences but there was a difference among sites. The total yields at sites 1, 2, and 3 were 77.86, 68.12, and 30.33 tonnes ha-1, respectively. Marketable yields were 59.04, 60.62, and 24.11 tonnes ha-1 at sites 1, 2, and 3, respectively. The lower yields were attributed primarily to less rainfall during July and August in 2001, and possibly to northern root-knot nematodes that were more prevalent at site 3. Mean levels of root-lesion nematodes in soil were highest (2690 nematodes kg-1) in carrot that followed timothy (Phleum pratense cv. Common), lowest (1100 nematodes kg-1) in carrots that followed marigold (Tagetes erecta cv. Crackerjack), and intermediate after barley (Hordeum vulgare cv. Chapais), pearl millet (Pennisetum glaucum cv. Millet 101), and annual ryegrass (Lolium multiflorum cv. Lemtal). Root-lesion nematode populations were also lower in marigold than in the other crops. Northern root-knot nematodes were not detected in rotation crops. The study indicated that carrot yields did not differ irrespective of the previous crop, but root-lesion nematode populations in soil at harvest were highest in carrots that followed timothy and lowest in carrots that followed marigolds. Population levels of root-knot nematodes in carrots did not differ among the crop sequences.
Résumé
Le rendement de la carotte (Daucus carota) et l’importance des populations du nématode des lésions de racines Pratylenchus penetrans et du nématode cécidogène du nord Meloidogyne hapla ont été mesurés dans cinq cultures utilisées en rotation et dans les cultures subséquentes de carottes à trois sites en champ (1998-1999, 1999-2000 et 2000-2001). La moyenne des rendements totaux et commercialisables pour les trois sites ne différait pas en fonction de la séquence des cultures, mais différait en fonction des sites. Les rendements totaux aux sites 1, 2 et 3 ont été respectivement de 77,86, 68,12 et 30,33 tonnes ha-1. Les rendements commercialisables ont été respectivement de 59,04, 60,62 et 24,11 tonnes ha-1 aux sites 1, 2 et 3. Les rendements les plus faibles ont été principalement attribués à des précipitations moins importantes au cours des mois de juillet et août 2001, et peut-être aussi à la présence du nématode cécidogène du nord au site 3. Les populations moyennes du nématode des lésions de racines ont été plus élevées (2690 nématodes kg-1) dans la carotte qui suivait la phléole (Phleum pratense cv. Common), plus faibles (1100 nématodes kg-1) dans la carotte qui suivait le tagète (Tagetes erecta cv. Crackerjack) et intermédiaires après l’orge (Hordeum vulgare cv. Chapais), le mil perlé (Pennisetum glaucum cv. Millet 101) et le ray-grass d’Italie (Lolium multiflorum cv. Lemtal). Les popu-lations du nématode des lésions de racines ont aussi été plus faibles dans les cultures de tagètes que dans les autres cultures. Le nématode cécidogène du nord n’a pas été détecté dans les cultures utilisées dans la rotation. L’étude a montré que le rendement de la carotte n’est pas influencé par la culture précédente, mais que les populations du nématode des lésions de racines dans le sol au moment de la récolte étaient plus élevées dans la carotte qui suit la phléole et plus faibles dans la carotte qui suit le tagète. L’importance de la population du nématode cécidogène du nord pour la carotte est indifférente à la séquence des cultures.
Article body
Introduction
The root-lesion nematode, Pratylenchus penetrans (Cobb) Filpjev & Schuurmans-Stekhoven, is prevalent in the Maritime Provinces (Kimpinski and Thompson 1990) and has caused signficant losses in potato (Solanum tuberosum L.) (Kimpinski 1982; Kimpinski et al. 2001), but the role of this root parasite in carrot (Daucuscarota L.) is unclear. However, root-lesion nematodes caused branching and reduced the size of carrot taproots in organic soils under greenhouse conditions (Vrain and Bélair 1981). Root-knot nematodes inflict much more serious damage worldwide (Potter and Olthof 1993), and the northern root-knot nematode, Meloidogyne hapla Chitwood, causes significant losses in Quebec (Bélair and Fournier 1997) and in localized areas of the Maritime provinces (Diamond et al. 1991; Kimpinski and Sturz 2000).
Chemicals to suppress nematodes are expensive, require specialized equipment, and do not always increase yields. These treatments also reduce populations of other soil organisms, including beneficial bacterial-feeding nematodes (Sturz and Kimpinski 1999). Furthermore, the environmental pollution and health hazards associated with pesticide use have increased the interest in alternative nematode management practices (Bélair 1992). As a result, several studies have indicated that some cropping sequences can reduce nematode populations and damage to the cash crop (Bélair and Parent 1996; Kimpinski et al. 2000). Previous work on Prince Edward Island also indicated that annual ryegrass (Lolium multiflorum Lam.) and wheat (Triticum aestivum L.) were less desirable hosts for root-lesion nematodes than red clover (Trifolium repens L.) and timothy (Phleum pratense L.) (Kimpinski and Willis 1980; Kimpinski et al. 1984). Pearl millet (Pennisetum glaucum R. Br.) is also a relatively poor host for root-lesion nematodes (Bélair et al. 2002; Kimpinski and Kunelius 2001).
The objectives of this study were to select several cover crops that were suitable for conditions in Prince Edward Island and the Maritime Provinces, and to assess their effects on yield of the subsequent carrot crop and on root-lesion nematodes. The incidence of the northern root-knot nematode was also monitored.
Materials and methods
Field trials
The project was conducted over 4 yr and involved three commercial sites in the Brookfield area of Prince Edward Island (lat. 46°21’ N, long. 63°9’ W). The soil type at each location was a fine sandy loam that averaged 70% sand, 20% silt, 10% organic matter, and a pH range of 5.2 to 6.2. These sites were typical carrot-producing fields found on Prince Edward Island and had annual ryegrass (cv. Lemtal) as the previous crop which was treated with glyphosate (N-(phosphonomethyl)glycine) in October of each yr. The sites were approximately 5 km from each other.
On 3 June 1998 at the first site, barley (Hordeum vulgare L., cv. Chapais), timothy (cv. Common), annual ryegrass (cv. Lemtal), pearl millet (cv. Millet 101), or marigold (Tagetes erecta L. cv. Crackerjack) were planted in 30.4 m X 9.1 m plots. The same five crops were planted on 30 June 1999 at the second site and on 29 June 2000 at the third site. Cultural and fertilizer practices similar to those used in commercial production were followed for barley, annual ryegrass and timothy (Anonymous 1991). The instructions on labels from suppliers were followed for pearl millet and marigold since these crops are not widely grown in the Atlantic region.
After the fall harvest of the rotation crops on 25 October 1998, 25 October 1999, and 5 October 2000 (except for barley which was harvested in early September in each yr), each site was split into 15.2 m X 9.1 m plots, which were either fall plowed or tilled the following spring. Prior to seeding carrot (cv. Neptune) in beds on 21 May 1999, 20 May 2000, and 29 May 2001, the sites were disced and harrowed. The beds were 18 cm across the top and 28 cm high. Seed placement was in a 3-line staggered arrangement with seed spacings of 72 seeds m-1 within lines and 3.8 cm between lines, and 86 cm between rows. Cultural and fertilizer practices similar to those used in commercial carrot production were followed (Anonymous 1992). Carrots were hand-harvested from 5 m lengths of each plot on 28 and 30 September 1999, 19 and 20 September 2000, and 10 October 2001. Unmarketable and total yields were recorded, and marketable yield was calculated from roots in the 19 - 44 mm crown root diam category using Canadian Standards (Canadian Food Inspection Agency 2003).
Nematode methodology
Nematode populations were determined in the rotation crop plots from soil on 3 June 1998, 30 June 1999, and 29 June 2000, as well as in soil and roots on 26 October 1998, 25 October 1999, and 5 October 2000. Spring soil samples in 1999 and 2001 and fall root and soil samples in 1999, 2000, and 2001 were collected from carrot plots on the same dates as for the rotation crops.
Numbers of nematodes in soil were estimated by removing in a random fashion 10 soil cores (25 mm diam, 15 to 20 cm in depth) from each plot. The soil cores in each sample were mixed thoroughly and a 50 g subsample was removed and placed in a modified Baermann funnel (Kimpinski 1993). Numbers of nematodes in roots were determined by placing up to 10 g of fresh secondary roots from each sample in a mist chamber (Hooper 1986). After 7 d at 22°C, nema-todes that had emerged from soil and roots were counted at 40-80X with a stereomicroscope. During the course of the field trials, at least 400 adult female root-lesion nematodes were examined with a compound microscope (1000 magnification) to confirm species identification. After the nematodes were extracted, roots and soil were dried for 24 h at 100°C, and counts were expressed as numbers g-1 of dry root and kg-1 of dry soil.
Field design and data analysis
The experimental designs in all cases were strip plots, also known as sub-unit treatments in strips (Cochran and Cox 1957), with four replicates for each treatment. The nematode data were transformed to log10(x+1) for analyses of variance and mean separation by Duncan’s multiple-range test (Genstat 2002; Snedecor and Cochran 1989). Since error variances were homogenous, yr was treated as a fixed effect and combined-year-analyses were conducted.
Results and discussion
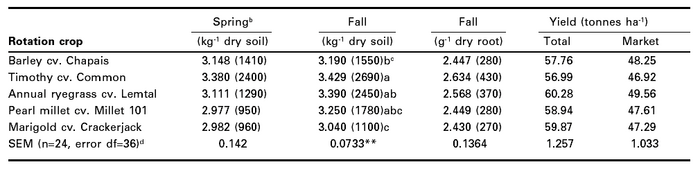
The average total and marketable carrot yield from the three sites did not differ (P < 0.05), irrespective of the previous rotation crop (Table 1). There was a significant (P < 0.001) site and/or yr effect. The mean total yield at site 1 in 1999 and at site 2 in 2000 were 77.86 and 68.12 tonnes ha-1 respectively, while at site 3 in 2001, the mean total yield was only 30.33 tonnes ha-1 (derived from Table 1). A similar trend occurred for marketable yield in 1999 and 2000 with 59.04 and 60.62 tonnes ha-1 respectively, but only 24.11 tonnes ha-1 in 2001 (derived from Table 1). The effects of the fall-plow and spring-plow treatments on carrot yield did not differ.
Root-lesion nematodes, consisting primarily of P. penetrans and few P. crenatus Loof, were the dominant plant-parasitic nematodes in root-zone soil and in roots of carrot and the rotation crops at the three sites (Tables 1 and 2). A few plots recorded northern root-knot nematode second-stage juveniles at sites 1 and 2, while at site 3, northern root-knot nematode juveniles were recovered from about half the carrot plots (data not presented in tables), at a mean level of approximately 3500 nematodes kg-1 of soil.
Although carrot yield did not differ in any of the crop sequences, the average number of root-lesion nematodes from the three sites in soil in the fall were most prevalent in carrot that followed timothy (2690 kg-1) and least prevalent in carrot that followed marigold (1100 kg-1) (P < 0.01) (Table 1). In rotational crops grown prior to carrots, the levels of root-lesion nema-todes in rhizosphere soil and in roots were also lower in marigold (P < 0.001) than in the other rotation crops (Table 2).
Table 1
Populations of root-lesion nematodesa in carrot and carrot yields following rotation crops (combined data from 1999, 2000 and 2001 at sites 1, 2 and 3, respectively)
a Primarily Pratylenchus penetrans.
b Based on 1999 and 2001 data only; n=16, error df=24
c Log10(x+1) transformed means with back-transformed means in parentheses; in a column, means followed by same letter are not different (P ≤ 0.05) according to Duncan’s multiple-range test; letters omitted if means are not different.
d Standard errors of the mean derived from log10(x+1) transformed data; ** indicated differences at P ≤ 0.01 according to the analysis of variance.
There were no differences in spring soil populations of root-lesion nematodes prior to planting the rotational crops or subsequent carrot crop (Tables 1 and 2). There was also no difference in the fall root populations of root-lesion nematodes in carrot (Table 1). There was a tillage effect (P < 0.001) on fall soil populations of root-lesion nematodes in carrots (1260 and 2620 nematodes kg-1 soil for fall and spring plowing, respectively; derived from Table 1). This was expected since fall plowing would destroy root systems and deplete food reserves, and also increase nematode desiccation. However, as indicated above, the difference in nematode populations caused by the different plowing times did not impact significantly on carrot yields. There were also site/year effects on root-lesion nematodes in carrot soil in the fall (1860, 1120, and 2880 nematodes kg-1 soil; P < 0.05), and in soil prior to seeding rotational crops (4080, 3330, and 11 380 nematodes kg-1 soil; P < 0.01) at sites 1, 2, and 3, respectively; derived from Tables 1 and 2).
Table 2.
Populations of root-lesion nematodesa in rotation crops (combined data from 1998, 1999 and 2000 at sites 1, 2 and 3, respectively)
a Primarily Pratylenchus penetrans.
b Log10(x+1) transformed means with back-transformed means in parentheses; in a column, means followed by same letter are not different (P _ 0.05) according to Duncan’s multiple-range test; letters omitted if means are not different.
c Standard errors of the mean derived from log10(x+1) transformed data; *** indicated differences at P _ 0.001 according to the analysis of variance.
The most obvious difference was that carrot yield was approximately 50% lower in 2001 at site 3 than in the two previous yr and sites. A plausible reason for the difference was the variation in precipitation during each yr. In 1999 and 2000, the total July- August precipitation at a weather station located about 5 km from the field sites was 260 and 228 mm, respectively, but only 65 mm in 2001. However, an added factor may have been the greater prevalence of northern root-knot nematodes that were recovered in the fall samples at site 3. In 1999 at site 1, northern root-knot nematodes were detected in carrot roots in three of the 40 plots, and at site 2 in 2000, no root-knot nematodes were detected. However, in 2001 at site 3, at harvest, northern root-knot nematodes were recovered from carrot roots in 23 plots with a mean count of 3470 g-1 of soil and a range of 430 to 41 020 g-1. These population levels are well above the ‘zero tolerance’ threshold in carrot fields outlined by Potter and Olthoff (1993), and higher than levels that reduced yields in field plots and growth chambers (Bélair 1984; Vrain 1982). As such, we speculate that the lack of rainfall and the greater numbers of northern root-knot nematode at site 3 in 2001 resulted in lower carrot yield.
Analysis of the data from site 3 did not indicate any differences in northern root-knot nematode populations in the different crop sequences. This was expected since the four rotation grasses were non-hosts for this nematode species, and marigold was also a very poor host in this study. Previous investigations have given variable results on the influence of marigold on root-lesion, as well as on northern root-knot nematodes. The observation that marigold cultivars were poorer hosts than the other rotation crops for root-lesion nematodes agreed with previous field studies in Connecticut (Miller and Ahrens 1969) and Prince Edward Island (Kimpinski et al. 2000). Kimpinski et al. (2000) observed that the numbers of root-lesion nematodes in mineral soils in field plots were lower and the tuber yields were higher when potato followed marigold than when the previous crop was red clover. However, Riga and Potter (1998) in a greenhouse study observed that populations of northern root-knot nematodes, but not root-lesion nematodes, were lower in tomato exposed to marigold root exudates. In contrast, Bélair (1992) working in organic field soils observed that northern root-knot nematodes actually increased in marigold, and root-gall indices in subsequent carrot crops were higher than in carrot following several other rotation crops. The conflicting results were probably due to different environmental conditions, different marigold cultivars, and different cash crop hosts.
Root-lesion nematodes reduced carrot growth and induced heavy branching of the taproots under greenhouse conditions (Vrain and Bélair 1981). The results of a survey in Quebec also indicated a high incidence of branched carrot roots in fields where root-lesion nematodes were prevalent (Vrain 1978). Nevertheless, root-knot nematodes usually inflict much more serious damage worldwide (Potter and Olthof 1993). The northern root-knot nematode, M. hapla, has caused significant losses in Quebec (Bélair and Four-nier 1997) and in localized areas of the Maritime provinces (Diamond et al. 1991; Kimpinski and Sturz 2000). In the Maritime region, non-host grass crops grown prior to carrot are a viable management practice for the suppression of the northern root-knot nematode, and crops such as red clover that are good hosts should be avoided. Marigold cultivars that have nema-tostatic and nematicidal properties may also be useful in reducing root-lesion nematode population levels.
Appendices
Acknowledgements
The authors thank the Prince Edward Island ADAPT Council for financial assistance. We also thank Brookfield Gardens, Brookfield, Prince Edward Island, and Sylvia Wyand, Claude Gallant and Janet McIsaac from Crops and Livestock Research Centre for advice and technical assistance.
References
- Anonymous. 1991. Atlantic Provinces field crop guide. Publication No. 100, Agdex 100.32, Atlantic Provinces Agricultural Services Coordinating Committee. Halifax, NS. 55 pp.
- Anonymous. 1992. Vegetable crops production guide for the Atlantic Provinces. Publication No. 1400, Agdex No. 250, Atlantic Provinces Agriculture Services Coordinating Committee. Summerside, PE. 52 pp.
- Bélair, G. 1984. Tolerance of carrot cultivars to northern root-knot nematode as influenced by preplant population densities. Phytoprotection 65 : 69-73.
- Bélair, G. 1992. Effects of cropping sequences on population densities of Meloidogyne hapla and carrot yield in organic soil. J. Nematol. 24 : 450-456.
- Bélair, G., and Y. Fournier. 1997. Plant bed treatment with 1,3-dichloropropene for Meloidogyne hapla control in carrots grown in organic soil. Phytoprotection 78 : 35-39.
- Bélair, G., and L.E. Parent. 1996. Using crop rotation to control Meloidogyne hapla Chitwood and improve marketable carrot yield. HortScience 31 : 106-108.
- Bélair, G., Y. Fournier, N. Dauphinais, and O.P. Dangi. 2002. Reproduction of Pratylenchuspenetrans on various rotation crops in Quebec. Phytoprotection 83 : 111-114.
- Canadian Food Inspection Agency. 2003. Fresh vegetable inspection manuals, Carrots. [Online] Available: http://www.inspection.gc.ca/english/plaveg/ fresh/vegleg/carrot/carrote.shtml. [27 Aug. 2003].
- Cochran, W.G., and G.M. Cox. 1957. Experimental designs. 2nd ed. John Wiley & Sons, New York. 611 pp.
- Diamond, J., J. Kimpinski, and C.E. Gallant. 1991. Root lesion and root-knot nematodes associated with crops grown in rotation with carrots on Prince Edward Island. Can. Plant Dis. Surv. 71 : 13-15.
- Genstat. 2002. Gentstat 6th Ed., Version 6.10.200 (PC Windows 2000), http://www.vsn-intl.com/ Committee of the statistics department, Rothamsted Experimental Station, Laws Agricultural Trust, VSN International Ltd. Oxford, UK.
- Hooper, D.J. 1986. Extraction of nematodes from plant material. Pages 51-58 in J.F. Southey (ed.), Laboratory methods for work with plant and soil nematodes. Reference Book 402, HMS0, London.
- Kimpinski, J. 1982. The effect of nematicides on Pratylenchus penetrans and potato yields. Am. Potato J. 59 : 327-335.
- Kimpinski, J. 1993. Nematodes. Pages 333-339 in M.R. Carter (ed.), Soil sampling and methods of analysis. Lewis Publishers, Boca Raton, FL.
- Kimpinski, J., and H.T. Kunelius. 2001. Forage millets for suppression of root lesion nematodes. AAFC Crops and Livestock Research Centre, Agri-Info, Factsheet 01-11 Agdex 628. Charlottetown, PE. 1 p.
- Kimpinski, J., and A.V. Sturz. 2000. Root-knot nematodes. Prince Edward Island Department of Agriculture and Forestry, Agdex 161/3. Charlottetown, PE. 2 pp.
- Kimpinski, J., and L.S. Thompson. 1990. Plant parasitic nematodes and their management in the Maritime provinces of Canada. Phytoprotection 71 : 45-54.
- Kimpinski, J., and C.B. Willis. 1980. Influence of crops in the field on numbers of root lesion and stunt nematodes. Can. J. Plant Pathol. 2 : 33-36.
- Kimpinski, J., H.T. Kunelius, and C.B. Willis. 1984. Plant parasitic nematodes in temperate forage grass and legume species in Prince Edward Island. Can. J. Plant Pathol. 6 : 160-164.
- Kimpinski, J., W.J. Arsenault, C.E. Gallant, and J.B. Sanderson. 2000. The effect of marigolds (Tagetes spp.) and other cover crops on Pratylenchus penetrans and on following potato crops. J. Nematol. 32 : 531-536.
- Kimpinski, J., W.J. Arsenault, and A.V. Sturz. 2001. Differential effect of nematicide treatments on tuber yields in early- and late-maturing potato cultivars. Plant Pathol. 50 : 509-514.
- Miller, P.M., and J.F. Ahrens. 1969. Influence of growing marigolds, weeds, two cover crops, and fumigation on subsequent populations of parasitic nematodes and plant growth. Plant Dis. Rep. 53 : 642-646.
- Potter, J.W., and T.H.A. Olthof. 1993. Nematode pests of vegetable crops. Pages 171-207 in K. Evans, D.L. Trudgill and J.M. Webster (eds.), Plant parasitic nematodes in temperate agriculture, CAB International, Wallingford, Oxon.
- Riga, E., and J.W. Potter. 1998. Marigolds as biological control agents of plant nematodes. Nematologica 44 : 568-569 (abstr.).
- Snedecor, G.W., and W.G. Cochran. 1989. Statistical methods. 8th ed. Iowa State University Press, Ames, Iowa. 503 pp.
- Sturz, A.V., and J. Kimpinski. 1999. Effects of fosthiazate and aldicarb on populations of plant- growth-promoting bacteria, root-lesion nematodes and bacteria-feeding nematodes in the root zone of potatoes. Plant Pathol. 48 : 26-32.
- Vrain, T.C. 1978. Dissémination et importance des nématodes phytoparasites dans les sols organiques du Québec. Phytoprotection 59 : 186 (abstr.).
- Vrain, T.C. 1982. Relationship between Meloidogyne hapla density and damage to carrots in organic soils. J. Nema-tol. 14 : 50-57.
- Vrain, T.C., and G. Bélair. 1981. Symptoms induced by the lesion nematode, Pratylenchus penetrans on carrot taproots in organic soil. Phytoprotection 62 : 79-81.
List of tables
Table 1
Populations of root-lesion nematodesa in carrot and carrot yields following rotation crops (combined data from 1999, 2000 and 2001 at sites 1, 2 and 3, respectively)
a Primarily Pratylenchus penetrans.
b Based on 1999 and 2001 data only; n=16, error df=24
c Log10(x+1) transformed means with back-transformed means in parentheses; in a column, means followed by same letter are not different (P ≤ 0.05) according to Duncan’s multiple-range test; letters omitted if means are not different.
d Standard errors of the mean derived from log10(x+1) transformed data; ** indicated differences at P ≤ 0.01 according to the analysis of variance.
Table 2.
Populations of root-lesion nematodesa in rotation crops (combined data from 1998, 1999 and 2000 at sites 1, 2 and 3, respectively)
a Primarily Pratylenchus penetrans.
b Log10(x+1) transformed means with back-transformed means in parentheses; in a column, means followed by same letter are not different (P _ 0.05) according to Duncan’s multiple-range test; letters omitted if means are not different.
c Standard errors of the mean derived from log10(x+1) transformed data; *** indicated differences at P _ 0.001 according to the analysis of variance.



 10.7202/706117ar
10.7202/706117ar