Abstracts
Abstract
Root rot caused by Fusarium oxysporum is a disease that reduces red clover persistence. Agronomical management of red clover includes MCPA application, and there is no information regarding the effects of this herbicide on the disease. MCPA was evaluated for its effects on F. oxysporum root rot and red clover (Trifolium pratense) growth in a greenhouse experiment. Additionally, in vitro mycelial growth and conidial germination of F. oxysporum were studied. For shoot dry weight and crown diameter of seedlings, the interaction of herbicide and inoculum was significant at 30 d. The herbicide–inoculum treatment reduced shoot dry weight by 20% at 1X rate and by 24% at 2X rate, and crown diameter was reduced by 10% at the high rate. The MCPA treatment caused a 40% reduction of root dry weight by the end of the experiment. Application of MCPA caused fusarium root rot to increase in severity on red clover seedlings and caused phytotoxicity at the high rate. Interaction with the other growth parameters was not significant, indicating that the effects of herbicide and inoculum were independent. Conidial germination and mycelial growth in vitro were reduced by MCPA. Results suggest that red clover growth could be negatively affected by F. oxysporum after MCPA application and that root rot severity increases at high rates of MCPA.
Keywords:
- Herbicide,
- legume,
- phytotoxicity,
- plant diseases
Résumé
La pourriture fusarienne des racines, causée par le Fusarium oxysporum, est une maladie qui diminue la pérennité du trèfle rouge. Les pratiques agronomiques pour la culture du trèfle rouge incluent l'application de MCPA, mais on ne connaît pas les effets de cet herbicide sur la maladie. Les effets du MCPA sur la pourriture fusarienne des racines, causée par le Fusarium oxysporum, et sur la croissance du trèfle rouge (Trifolium pratense) furent évalués lors d'une expérience en serre. De plus, la croissance mycélienne in vitro et la germination des conidies du F. oxysporum furent étudiées. Pour le poids de matière sèche du système foliacé et pour le diamètre du collet des plantules, l'interaction de l'herbicide et de l'inoculum était significative à 30 jours. Le traitement herbicide–inoculum diminua le poids de matière sèche du système foliacé de 20 % à une fois la dose et de 24 % à deux fois la dose, et le diamètre du collet fut réduit de 10 % à la dose la plus élevée. À la fin de l'expérience, le traitement au MCPA avait réduit de 40 % le poids de matière sèche des racines. L'application de MCPA a fait augmenter l'intensité de la pourriture fusarienne des racines sur les plantules de trèfle rouge et a provoqué de la phytotoxicité à forte dose. L'interaction avec les autres paramètres de la croissance n'était pas significative, ce qui indique que l'effet de l'herbicide et celui de l'inoculum étaient indépendants. Le MCPA a réduit la germination des conidies et la croissance mycélienne in vitro. Les résultats suggèrent que la croissance du trèfle rouge pourrait être affectée négativement par le F. oxysporum après l'application de MCPA et que l'intensité de la pourriture fusarienne des racines augmente avec de fortes doses de MCPA.
Mots clés:
- Herbicide,
- légumineuse,
- maladies des plantes,
- phytotoxicité
Article body
Introduction
The use of herbicides in the production of field crops is an essential component of mechanized farming. Although increasing numbers of plant diseases are reported to occur more frequently or with greater severity after the application of some herbicides (Altman and Campbell 1977; Altman and Rovira 1989; Bollen 1993; Sanogo et al. 2000), yet other herbicides have been shown to cause decreases or no significant changes in plant diseases (Dann et al. 1999; Dissanayake et al. 1998). The incidence and severity of disease caused by fungal pathogens may be affected by the interaction of herbicides, plants, pathogens and other microorganisms (Heydari and Misaghi 2003; Heydari et al. 1997).
Red clover (Trifolium pratense L.) is an important perennial forage crop in Chile. Although it is considered a perennial species, it seldom persists beyond 2 or 3 yr. Fungal pathogens, particularly root diseases caused by species of Fusarium, are major components of the disease complex that impacts red clover persistence. Fusarium oxysporum Schlechtend. is the most common species and is the most prevalent pathogen recovered from diseased red clover plants (Steiner and Alderman 1999; Venuto et al. 1995). Fusarium-affected plants may exhibit poor or slow emergence, and the resulting seedlings are often stunted and weak. The pathogen penetrates directly through the epidermis or indirectly through hypocotyls' stomata, lenticels, or through wounds arising during secondary root formation, and thereby causes necrotic lesions on lower stems and roots (Carson et al. 1991).
The commercial formulation of MCPA ((4-chloro2-methylphenoxy) acetic acid), the ammonium salt, is widely used in Chile and worldwide (AFIPA 2002; Kudsk and Streibig 2003; Liu et al. 1994) to control broadleaf weeds in red clover. MCPA is absorbed by roots and foliage and is translocated into the plants to cause the death of the susceptible weeds. It causes uncontrolled growth of the meristematic tissues and restrains synthesis of DNA and proteins, thus disrupting basic metabolic processes in plant cells and tissues. While these effects are lethal to weeds, they may affect crops depending on a number of factors (Conrad and Stritzke 1980). Research on cereal crops has indicated that low concentrations of chlorinated phenoxyacetic acid derivatives may stimulate the rate of root and shoot growth, seed germination and photosynthesis, but the higher concentrations inhibit these processes (Grabinska-Sota et al. 2003; Hess 1993). MCPA causes stunting, distortion and gall formation on the roots of a number of crops (Tharp and Kells 2000). Studies on the effects of the most frequently used post-emergence herbicides, including MCPA presently used for weed control on red clover in Chile, indicated that this herbicide had the most detrimental effects on red clover plants (Ceballos et al. 2004).
There is no information available on the effects of MCPA on F. oxysporum, the causal agent of red clover root rot, and it is essential to examine the possible implications that such interactions may have for the successful management and stand life of red clover. The objective of this study was to assess the effects of MCPA on fusarium root rot and red clover growth under controlled greenhouse conditions.
Materials and Methods
An isolate of F. oxysporum was collected from diseased red clover plants at the Carillanca Experimental Station in Temuco, Chile. Specific characterization of this strain as F. oxysporum (IMI 390980) was provided by CABI Bioscience Identification Services, UK Centre (Egham). The pathogenicity of this isolate was proved by Koch’s postulates, and it was maintained on potato dextrose agar (PDA) and used in all experiments. The commercial formulation (MCPA ammonium salt 750 LS) of the herbicide MCPA was used.
Greenhouse studies of the MCPA–Fusarium oxysporum interaction
The experiment consisted of three rates of MCPA (0, 750 and 1500 g a.i. ha-1) corresponding to 0%, 100% (1X) and 200% (2X) of the recommended application rate for red clover. The herbicide was applied with a compressed-air bicycle sprayer at a pressure of 207 kPa, providing a volume of 200 L ha-1 of sterile water solution. The inoculum consisted of 5 mL of a 2.5 x 106 spores mL-1 conidial suspension of F. oxysporum, prepared from 2-wk-old cultures grown on PDA. Experimental units consisted of single plastic pots, 12 cm in diam, filled with microwave-sterilized soil (Islam and Weil 1998) that was planted with two red clover seeds, cv. Quiñequeli, previously disinfected for 5 min with a 0.5% sodium hypochlorite solution. Allophanic soil (pH 6.1, 17% organic matter, 32% sand, 53% silt, 15% clay) was used. Plants were fertilized at seedling establishment. Soil of each pot was fertilized with 200 mg P kg-1 soil (as Triple superphosphate) and 80 mg K kg-1 soil (as Sulpomag) before planting, and 50 mg N kg-1 soil (as Urea) was applied after emergence of plants.
Fungal inoculum was applied 15 d before the herbicide treatments (at one to two trifoliate leaf stage of plant growth) by injection into the root zone of the aseptically grown plants. Control plants were not inoculated. Herbicide was applied to seedlings at the two to three trifoliate leaf stage. Temperature was 20-25°C between night and day, and natural light was supplemented with a 16 h photoperiod and light incident of 3800 lx. Pots were watered regularly on the surface throughout the experimentation to ensure adequate soil moisture. The greenhouse experiment was a randomized complete design with 10 replications and was repeated once. F tests were done to determine homogeneity of error variances among the two tests (Sanogo et al. 2000), and data were pooled across the two tests accordingly for final analyses.
Seedlings were harvested at 20 and 30 d after herbicide treatment and washed under running tap water. Root length, seedling height, crown diam and dry weight of shoots and roots were measured. Harvested plants were checked for the presence of fungi, and the pathogen was reisolated from symptomatic tissues, completing Koch’s postulates.
Root rot disease was assessed on a scale of 1 to 4, where 1 = completely healthy root tissue; 2 = a few superficial dark brown lesions of root tissue; 3 = extended superficial dark brown lesions of root tissue; and 4 = necrotic root or tissue death. Shoot damage was assessed on a scale of 1 to 5, where 1 = no visible damage; 2 = some chlorotic leaves, up to 25%; 3 = 25% to 50% chlorotic leaves; 4 = > 50% chlorotic and some necrotic leaves; 5 = complete plant death. The root disease severity index (RDSI) and foliage damage index (FDI) were calculated using the formula ∑ (g x n)/N where g is the damage score, n is the number of seedlings assigned to each class, and N is the total number of plants (Vettraino et al. 2003).
Effects of MCPA on mycelial growth and conidial germination of F. oxysporum in vitro
To evaluate the effects of MCPA on growth, potato dextrose agar (PDA, 39 g L-1, Difco) was amended with the commercial formulation of the herbicide at 0, 1X and 2X rates. Final concentrations of MCPA per plate for the 1X and 2X rates were 24 µ mL-1 and 48 µ mL-1, respectively. Each solution of MCPA was added to cooled molten medium, mixed, and 20 mL of the medium was poured into Petri plates (9 cm diam). About 3 h after the medium was poured, a mycelial disk (5 mm in diam) taken from the periphery of a 2-wk-old culture of F. oxysporum growing on PDA was transferred to each herbicide-amended plates, and incubated at 22°C under continuous darkness. Radial growth of mycelium from the centre was recorded starting on d 2, and at 5 d intervals for 1 mo. Each treatment was replicated six times and treatments were arranged in a complete randomized design. Data of mycelial growth are given as a growth index obtained by dividing average diam growth for the treatment by that for the corresponding control.
For conidial germination study, conidia were produced by transferring a 5 mm plug stock culture onto PDA in 9 cm diam Petri plates and placing the plates in an incubator under continuous darkness at 22°C. After 2 wk, conidia were harvested by adding 15 mL of sterile distilled water per plate and gently scraping the medium surface with a soft paintbrush. The number of conidia was determined with a hemacytometer. To study the effects of MCPA on conidial germination, conidia were suspended in sterile distilled water (control) or in a solution of each rate (0, 1X and 2X) of the herbicide on Petri plates. The plates were sealed with Parafilm and incubated in the dark at 22°C for 6 h. The number of observed and germinated conidia was determined with a hemacytometer (Sanogo et al. 2000). The percentage of germinated conidia was computed as the ratio of germinated conidia to observed conidia. Each treatment was replicated six times and arranged in a complete randomized design.
Data analysis
Root length, seedling height, crown diam and dry weight of shoots and roots were subjected to a variance analysis and a Tukey test at a 5% significance level. Since foliage damage and root rot severity were evaluated using scales, resulting in ordinal data, these were analyzed using non-parametric statistic procedures (Ceballos et al. 2004; Shah and Madden 2004) by means of the Kruskal-Wallis test and groups were separated using the Conover-Inman test (P ≤ 0.05) (Conover 1999). Data of mycelial growth were analyzed by Dunnet multiple comparisons with a control test (P ≤ 0.05). Conidial germination data were analyzed by the Tukey test (P ≤ 0.05).
Results and Discussion
MCPA rate–inoculum interaction effects on red clover growth
For plant height, root length and root dry weight, the MCPA–inoculum interaction was not significant (Table 1), indicating that the effects of these factors were independent of each other during the experiment.
Table 1
Significance levels of the effects of three rates of MCPA herbicide and two inoculum levels of Fusarium oxysporum on growth parameters of red clover in two greenhouse trials
Plants treated with 2X rate of MCPA had 13% and 7% reduction in plant height at 20 and 30 d, respectively, compared with the untreated control (Table 2). Root length of red clover did not significantly differ among treatments throughout the experimental period. At 20 d, root dry weight was 18% and 27% lower in the 1X and 2X treatments, respectively, compared with the untreated control. The effect of the 1X rate was not statistically significant. At 30 d, the 1X and 2X rates significantly reduced root dry weight by 37% and 40%, respectively, but there were no significant differences between the rates.
Table 2
MCPA effect on plant height, root length and root dry weight of red clover plants
a Rate 1X: recommended rate; rate 2X: twice the recommended rate.
b For each sampling time period, within a column, values followed by the same letter are not significantly different based on Tukey’s test (P ≤ 0.05).
The interaction of MCPA–Fusarium for shoot dry weight and crown diam was significant, indicating that MCPA rates and inoculum levels were not independent of each other at 30 d (Table 1). The combination of herbicide–inoculum treatments reduced shoot dry weight by 20% and 24% at the 1X and 2X rates, respectively, in comparison with the inoculated plants (Table 3). Crown diam was reduced by 10% at the 2X rate compared with the inoculated plants. At 20 d, the MCPA–inoculum interaction was not significant (data not shown). Inoculated plants did not show significant differences compared with untreated plants at the end of the experiments.
Table 3
MCPA rate–inoculum level interaction effects on red clover crown diam and shoot dry weight at 30 d
a Rate 1X: recommended rate; rate 2X: twice the recommended rate.
b Values followed by the same letter are not significantly different based on Tukey’s test (P ≤ 0.05).
Root rot severity and foliage damage
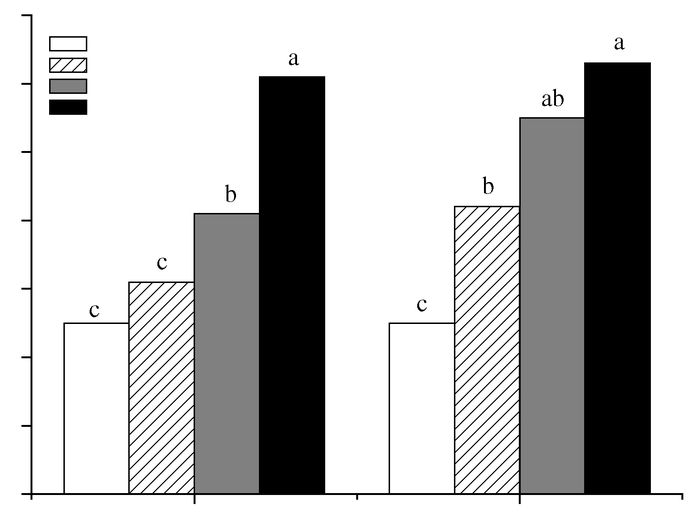
Root rot severity and foliage damage data from rating categories of plants were analyzed using the non-parametric Kruskal-Wallis test. Graphic presentation data are given by a root disease severity index (RDSI) (Fig. 1) and a foliage damage index (FDI) (Fig. 2).
Figure 1
Root disease severity index from MCPA–Fusarium oxysporum treatments on red clover plants.
Root rot severity was assessed on a scale of 1 to 4, where 1 = healthy and 4 = necrotic root.
a Treatments: Control: untreated; F0: Fusarium inoculum only; F0/1X: F0 + MCPA at 1X rate; F0/2X: F0 + MCPA at 2X rate. For each time period, different letters indicate that means are significantly different based on Conover-Inman’s test (P ≤ 0.05).
Application of MCPA significantly increased fusarium root rot severity in red clover seedlings at the 1X and 2X rates at 20 d (Fig. 1). At 30 d, RDSI increased and significant differences were observed only at the 2X rate compared with inoculated plants. MCPA had phytotoxic effects (droopiness, flaccidity, chlorotic and necrotic leaves) on the foliage of inoculated plants at the 2X rate. At 30 d, FDI showed significant differences between inoculated and non-inoculated plants, but no rate effect was detected (Fig. 2). Both RDSI and FDI showed significant differences between inoculated and non-inoculated plants at 30 d.
Figure 2
Foliage damage index from MCPA–Fusarium oxysporum treatments on red clover plants.
Foliage damage was assessed on a scale of 1 to 5, where 1 = no visible damage and 5 = plant death.
a Treatments: Control: untreated; F0: Fusarium inoculum only; F0/1X: F0 + MCPA at 1X rate; F0/2X: F0 + MCPA at 2X rate. For each time period, different letters indicate that means are significantly different based on Conover-Inman’s test (P ≤ 0.05).
Mycelial growth and conidia germination of Fusarium oxysporum in vitro
Mycelial growth at both rates of MCPA showed no differences from the control although some inhibition was observed for the 1X rate at the beginning and at the end of the experiment. At 2 and 27 d, mycelial growth was 16% and 20% lower than the control, respectively (Fig. 3). The variable responses in fungitoxic effect of MCPA for rate 1X might have resulted from a modification of the nutrient component of media with toxic substances from the degradation of herbicides (Liu et al. 1997). For conidial germination study, MCPA significantly reduced the germination of conidia by 24% when used at the 2X rate compared with untreated conidia, but the 1X rate had no effect.
Figure 3
Mycelial growth of Fusarium oxysporum on PDA amended with MCPA in vitro.
Data are given as a growth index obtained by dividing average diam of growth for the treatment by average diam of growth for the corresponding control.
a 1X: Recommended rate; 2X: twice the recommended rate.
* Indicates significantly different from the control, based on Dunnet multiple comparisons (P ≤ 0.05).
The effects of herbicides on disease development generally result from interactions between direct effects on the pathogen and indirect effects via plant-mediated responses (Altman and Rovira 1989; Bollen 1993). The present study showed that when the direct effects of herbicide on F. oxysporum were examined, MCPA inhibited spore germination, at the high rate, and had some inhibitory effect on mycelial growth, especially at the MCPA rate of 1X. Other studies with Fusarium spp. have also shown inhibitory responses (Grossbard 1976), but stimulation of growth of Fusarium spp. by some herbicides has been reported (Mussa and Russell 1977). The effect of a herbicide on disease levels is not always the same as its effect on the pathogen in vitro study (Sanogo et al. 2000), because direct contact between the pathogen and the herbicide would not be as likely to occur in the complex interaction under natural environmental conditions. Some studies reported that Fusarium growth is dependent on the nutrient content of media (Liu et al. 1997; Sanogo et al. 2000), and this could represent different nutritional levels in plant tissue, as influenced by indirect herbicide effects on plants (Altmann and Rovira 1989; Jeffery and Burgess 1990). This study was carried out at the optimum nutritional conditions for mycelial growth of F. oxysporum (Sanogo et al. 2000).
In the present study, MCPA application to red clover plants inoculated with F. oxysporum resulted in a reduction of shoot growth and crown diam. Although no herbicide–inoculum interactions was observed for plant height and root dry weight, these factors were reduced by MCPA. Additionally, MCPA caused fusarium root rot to increase in severity on red clover seedlings at high rates, and also caused foliar damage.
This study also showed that seedling injury from exposure to MCPA was evident in the absence of the pathogen. On the shoots, symptoms included leaf epinasty, stem curvature and leaf chlorosis and necrosis. Symptoms of root injury included visible cracking and localized necrosis. Such symptoms have been previously reported (Ceballos et al. 2004; Clay 1989; Devine et al. 1993; Grossmann 2000). The root cracks particularly appeared to be favourable sites for ingress by the pathogen because lesions typically occurred at such cracks. FDI was defined based on the phytotoxicity effect of the herbicide on red clover. For RDSI, it was difficult to distinguish tissue injury caused by F. oxysporum from that caused by direct herbicide injury on the roots. Therefore, the presence of infection was verified by incubating fragments of injured roots on PDA.
It has been reported that stress imposed by some herbicides can indirectly lead to increased disease in crops and that their application weakens and predisposes plants to fungal colonization (Bollen 1993; Carson et al. 1991). In the present study, MCPA affected the development of both inoculated and non-inoculated red clover plants and could explain the significant increases in disease severity compared with untreated plants. It is clear that MCPA and Fusarium were not independent in their effects on plant development.
The effect of MCPA on infection by F. oxysporum has not been previously reported, although comparisons can be made to results obtained with other herbicides, where pathogenicity of Fusarium was increased (Greaves 1989).
Based on data obtained from this study, MCPA can increase fusarium root rot severity in red clover seedlings in a controlled greenhouse environment. However, these results require verification under field conditions where many other factors may influence disease severity as well as the interaction between pathogens, herbicides, and the host plant.
Appendices
Aknowledgements
Financial support for this research was provided by the FONDECYT 1020297 and DIUFRO 120522 Projects.
References
- AFIPA. 2002. Manual Fitosanitario. Asociación de distribuidores de plaguicidas (ed.), Santiago, Chile. 677 pp.
- Altman, J., and C.L. Campbell. 1977. Effect of herbicides on plant diseases. Annu. Rev. Phytopathol. 15 : 361-385.
- Altman, J., and A.D. Rovira. 1989. Herbicide-pathogen interactions in soil-borne root diseases. Can. J. Plant Pathol. 11 : 166-172.
- Bollen, G.J. 1993. Mechanisms involved in nontarget effects of pesticides on soil-borne pathogens. Pages 281-301 in J. Altman (ed.), Pesticide interactions in crop production: beneficial and deleterious effects. CRC Press, Boca Raton, FL.
- Carson M.L., W.E. Arnold, and P.E. Todt. 1991. Predisposition of soybean seedlings to Fusarium root-rot with trifluralin. Plant Dis. 75 : 342-347.
- Ceballos, R., G. Palma, H. Brevis, F. Ortega, and A. Quiroz. 2004. The effect of five postemergence herbicides on red clover shoot and root growth in greenhouse studies. Phytoprotection 85 : 153-160.
- Clay, D. 1989. Herbicides, plant roots and crop performance. Asp. Appl. Biol. (Roots and the Soil Environments) 22 : 191-197.
- Conover, W.J. 1999. Practical nonparametric statistics. 3rd Ed. Wiley, New York, Chichester. 584 pp.
- Conrad, J.D., and J.F. Stritzke. 1980. Response of arrow leaf clover to postemerge herbicides. Agron. J. 72 : 670-672.
- Dann, E.K., B.W. Diers, and R. Hammerschmidt. 1999. Suppression of Sclerotinia stem rot of soybean by lactofen herbicide treatment. Phytopathology 89 : 598-602.
- Devine, M., S.O. Duke, and C. Fedtke. 1993. Physiology of herbicide action. Prentice Hall, Englewood Cliffs, NJ. p. 251-294.
- Dissanayake, N., J.W. Hoy, and J.L. Griffin. 1998. Herbicide effects on sugarcane growth, Pythium root rot, and Pythium arrhenomanes. Phytopathology 88 : 530-535.
- Grabinska-Sota, E., E. Wisniowska, and J. Kalka. 2003. Toxicity of selected synthetic auxines - 2,4-D and MCPA derivatives to broad-leaved and cereal plants. Crop Prot. 22 : 355-360.
- Greaves, M.P. 1989. Herbicide interactions with soil micro-organisms. Asp. Appl. Biol. (Roots and the Soil Environments) 22 : 405-412.
- Grossbard, E. 1976. Effects on the soil microflora. Pages 99-147 in L.J. Audus (ed.), Herbicides: physiology, biochemistry, ecology. 2nd Ed. Academic Press, London.
- Grossmann, K. 2000. Mode of action of auxin herbicides: a new ending to a long, drawn out story. Trends Plant Sci. 5 : 506-508.
- Hess, F.D. 1993. Herbicide effects on plant structure, physiology, and biochemistry. Pages 13-36 in J. Altman (ed.), Pesticide interactions in crop production: beneficial and deleterious effects. CRC Press, Boca Raton, FL.
- Heydari, A., and I.J. Misaghi. 2003. The role of rhizosphere bacteria in herbicide-mediated increase in Rhizoctonia solani-induced cotton seedling damping-off. Plant Soil 257 : 391-396.
- Heydari, A., I.J. Misaghi, and W.B. McCloskey. 1997. Effects of three soil-applied herbicides on populations of plant disease suppressing bacteria in the cotton rhizosphere. Plant Soil 195 : 75-81.
- Islam, K.R., and R.R. Weil. 1998. Microwave irradiation of soil for routine measurement of microbial biomass carbon. Biol. Fertil. Soils 27 : 408-416.
- Jeffery, S., and L.W. Burgess. 1990. Growth of Fusarium graminearum Schwabe group-1 on media amended with atrazine, chlorsulfuron or glyphosate in relation to temperature and osmotic potential. Soil Biol. Biochem. 22 : 665-670.
- Kudsk, P., and J.C. Streibig. 2003. Herbicides a two edged sword. Weed Res. 43 : 90-102.
- Liu, S.H., W.A. Quick, A.I. Hsiao, and J.C. Streibig. 1994. Effect of MCPA on the phytotoxicity of imazamethabenz-methyl applied to wild oats (Avena fatua L). Weed Res. 34 : 425-431.
- Liu, L., Z.K. Punja, and J.E. Rahe. 1997. Altered root exudation and suppression of induced lignification as mechanisms of predisposition by glyphosate of bean roots (Phaseolus vulgaris L.) to colonization by Pythium spp. Physiol. Mol. Plant Pathol. 51 : 111-127.
- Mussa, A., and P. Russell. 1977. The influence of pesticides and herbicides on the growth and virulance of Fusarium solani f. sp. phaseoli. J. Agric. Sci. Comb. 88 : 705-709.
- Sanogo, S., X.B. Yang, and H. Scherm. 2000. Effects of herbicides on Fusarium solani f. sp. glycines and development of sudden death syndrome in glyphosate-tolerant soybean. Phytopathology 90 : 57-66.
- Shah, D.A., and L.V. Madden. 2004. Nonparametric analysis of ordinal data in designed factorial experiments. Phytopathology 94 : 33-43.
- Steiner, J.J., and S.C. Alderman. 1999. Red clover seed production. V. Root health and crop productivity. Crop Sci. 39 : 1407-1415.
- Tharp, B.E., and J.J. Kells. 2000. Effect of soil-applied herbicides on establishment of cover crop species. Weed Technol. 14 : 596-601.
- Venuto, B.C., R.R. Smith, and C.R. Grau. 1995. Virulence, legume host-specificity, and genetic relatedness of isolates of Fusarium oxysporum from red-clover. Plant Dis. 79 : 406-410.
- Vettraino, A.M., A. Belisario, M. Maccaroni, and A. Vannini. 2003. Evaluation of root damage to English walnut caused by five Phytophthora species. Plant Pathol. 52 : 491-495.
List of figures
Figure 1
Root disease severity index from MCPA–Fusarium oxysporum treatments on red clover plants.
Root rot severity was assessed on a scale of 1 to 4, where 1 = healthy and 4 = necrotic root.
a Treatments: Control: untreated; F0: Fusarium inoculum only; F0/1X: F0 + MCPA at 1X rate; F0/2X: F0 + MCPA at 2X rate. For each time period, different letters indicate that means are significantly different based on Conover-Inman’s test (P ≤ 0.05).
Figure 2
Foliage damage index from MCPA–Fusarium oxysporum treatments on red clover plants.
Foliage damage was assessed on a scale of 1 to 5, where 1 = no visible damage and 5 = plant death.
a Treatments: Control: untreated; F0: Fusarium inoculum only; F0/1X: F0 + MCPA at 1X rate; F0/2X: F0 + MCPA at 2X rate. For each time period, different letters indicate that means are significantly different based on Conover-Inman’s test (P ≤ 0.05).
Figure 3
Mycelial growth of Fusarium oxysporum on PDA amended with MCPA in vitro.
Data are given as a growth index obtained by dividing average diam of growth for the treatment by average diam of growth for the corresponding control.
a 1X: Recommended rate; 2X: twice the recommended rate.
* Indicates significantly different from the control, based on Dunnet multiple comparisons (P ≤ 0.05).
List of tables
Table 1
Significance levels of the effects of three rates of MCPA herbicide and two inoculum levels of Fusarium oxysporum on growth parameters of red clover in two greenhouse trials
Table 2
MCPA effect on plant height, root length and root dry weight of red clover plants
Table 3
MCPA rate–inoculum level interaction effects on red clover crown diam and shoot dry weight at 30 d







 10.7202/010907ar
10.7202/010907ar