Abstracts
Abstract
Seasonal variation in relative Blueberry scorch virus (BlScV) concentration was determined for three infected highbush blueberry, Vaccinium corymbosum, cultivars in a commercial field in southwestern British Columbia, Canada. Relative virus concentration per g of infected blueberry flower clusters and leaf terminal tissue varied during the season with significant cultivar-by-time interactions. Flower clusters had the highest BlScV concentration per g of tissue and could be collected in early May for disease surveys. Timing of leaf sample collection for BlScV surveys, transmission studies and virus purification should be based on studies of temporal variation in BlScV concentration for the principal cultivars in a production area.
Keywords:
- Blueberry scorch virus,
- DAS-ELISA,
- highbush blueberry,
- virus concentration
Résumé
Les variations saisonnières dans la concentration relative du virus de la brunissure nécrotique du bleuet (Blueberry scorch virus; BlScV) ont été déterminées pour trois cultivars infectés du bleuetier géant, Vaccinium corymbosum, dans un champ commercial du sud-ouest de la Colombie-Britannique, Canada. La concentration relative du virus par g de grappes de fleurs et de tissu terminal de feuille infectés a varié au cours de la saison, avec des interactions significatives entre les cultivars et le temps. Les grappes de fleurs avaient la plus haute concentration du virus par g de tissu et pouvaient être cueillies au début de mai pour évaluer la maladie. Le moment choisi pour la collecte des échantillons de feuille pour l’étude du BlScV, les études de transmission et le traitement du virus devrait se fonder sur des études de variation temporelle dans la concentration du BlScV pour les principaux cultivars d’une zone de production.
Mots clés:
- concentration virale,
- bleuetier géant,
- test ELISA sandwich,
- virus de la brunissure nécrotique du bleuet
Article body
Introduction>
Blueberry scorch virus (BlScV), a Carlavirus, has been found in highbush blueberry, Vaccinium corymbosum L., in North America (Bristow et al. 2000; Cavileer et al. 1994; Martin and Bristow 1988) and Europe (Ciuffo et al. 2005). The virus can cause blighting of stems, leaves and flowers, and significant yield reductions in susceptible cultivars (Bristow et al. 2000). Symptom expression also varies with virus strain (Wegener et al. 2006). BlScV is aphid-borne and has a non-persistent mode of transmission (Bristow et al. 2000). Control of the aphid vectors and early detection and removal of infected plants is recommended to manage BlScV (British Columbia Ministry of Agriculture and Lands 2007).
The epidemiology of BlScV depends on several factors, including the population dynamics and transmission efficiency of aphid vectors, the number of infected plants within a field, and virus concentration in the infected plants. Ericaphis fimbriata (Richards) (=Fimbriaphis fimbriata) (Remaudière and Remaudière 1997) [Hemiptera: Aphididae], the dominant colonizing aphid on highbush blueberry in southwestern British Columbia, Canada (Raworth 2004), has been regarded as the most important vector of BlScV in studies conducted in Washington, USA (Bristow et al. 2000), although non-colonizing species may also be involved (Lowery et al. 2008). Ericaphis fimbriata has a consistent population pattern among years and locations; populations increase exponentially throughout the spring to reach a peak of 0.8 to 16.1 aphids per leaf terminal (300 to 9000 aphids per bush) in late June or early July, and then decline rapidly due to reduced plant quality for the aphids and natural enemies (Raworth 2004; Raworth and Schade 2006). More than 80 species of winged migrant aphids have been found in commercial blueberry fields in southwestern British Columbia (Raworth et al. 2006); however, spatial and temporal numerical patterns often depend on field, year, season and species peculiarities. Ericaphis fimbriata is not an efficient vector and, therefore, aphid numbers are important in virus transmission (Bristow et al. 2000). Nothing is known of the seasonal variation in BlScV concentration but, given the importance of aphid numbers, the synchrony between aphid population dynamics and temporal changes in BlScV concentration may affect the epidemiology. Fluctuations in concentration of other viruses at different stages of crop development have been reported in garlic (Dovas et al. 2002), red spruce (Bachand and Castello 1998), and peach (Dal Zotto et al. 1999; Polák 1995, 1998).
The purpose of the current work was to determine 1) the temporal variation in BlScV concentration in infected highbush blueberry cultivars in the field during the growing season; 2) whether or not the variation is consistent among cultivars; and 3) the implications of the findings for disease management and the timing of BlScV surveys, aphid transmission studies and virus purification.
Materials and methods
Two ‘Dixi’, two ‘Jersey’ and two ’Rubel’ bushes, all BlScV positive, approximately 2 m tall, and located near the epicentre of an infected area in a commercial field in Richmond, British Columbia, Canada, were sampled in 2007. The plants were more than 15 yr old, and had probably become infected with BlScV between 1989 (MacDonald and Martin 1990) and 2001 (Field 2-Richmond (Wegener et al. 2006)). Ten flower buds or clusters were collected - without reference to symptoms or other physical characteristics - from different parts of a bush every 2 wk between 14 March and 15 May (five sample dates for ’Dixi’ and ’Jersey’, and four for ’Rubel’), and 10 leaf buds or terminals (all leaves and stem arising from a bud) were collected between 2 April and 11 September (11 sample dates). Each bud or terminal was stored in a separate plastic bag. Samples were kept on ice in the field and then at -20°C until processing.
The samples were tested for BlScV in November 2007 using double antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) according to Clark and Adams (1977), except that the grinding buffer followed that of Martin and Bristow (1988). The entire bud or terminal was weighed and then homogenized in buffer. Sample weight to buffer volume ratio (g:ml) was held constant at 1:10 so that the quantity of sample extract from the small buds would be sufficient for DAS-ELISA tests. Samples from 14 March to 19 June were homogenized using a pestle and mortar, which was washed thoroughly under running water between samples. The remaining samples were homogenized in a wet-dry grinder (Revel CCM101, Revel Inc., Houston, TX, USA). Samples were analyzed using DAS-ELISA with optical density (OD) readings at 1 h, 2 h, and overnight (Phyto Diagnostics Co. Ltd., North Saanich, BC, Canada). Data needed to convert OD readings to relative virus concentration were obtained by simultaneously analyzing serial dilutions (10x, 20x, 40x, 80x and 160x) of extract from three of the samples.
Plant samples were considered infected if the OD readings were three or more times greater than the mean of the negative plate controls (Pataky et al. 2004; Sutula et al. 1986). Because virus concentration was the variable of interest, and it was not a linear function of the OD readings, relative virus concentration was calculated for each BlScV-positive OD reading prior to analysis using a relationship of the form y = e(a + bx), where y was relative virus concentration [(1/ dilution) * 1000] and x was OD reading. The 2 h OD readings provided the greatest r 2 (0.96) for the serial dilution data and a mean intercept (a = 0.40 ± 0.21 SE, Fig. 1A); b (2.52) was then calculated by fitting the equation to pass through the maximum OD reading in the data set (1.67) at the relative concentration of 100 [(1/10) * 1000] (Fig. 1B). This approach allowed comparisons of relative virus concentration among cultivars and sample dates.
Figure 1
Serial dilution data for three samples (circles, crossed circles, dark circles), with regressions (A), and the relationship used to convert DAS-ELISA OD readings to relative virus concentration (B).
Statistical analyses were performed on means and proportions calculated from the 10 samples collected from each replicate plant at every sample date: mean relative BlScV concentration per g of infected plant tissue (i.e. samples with OD readings less than three times the negative controls were deleted before calculating the mean); mean relative BlScV concentration per infected bud, flower cluster or leaf terminal obtained by multiplying the relative concentration per g of tissue by the tissue weight; and the proportion of infected samples. The data were analyzed by repeated measures ANOVA (PROC-GLM, SAS Institute Inc., Cary, NC, USA) to determine the effect of cultivar (2,3 df for flower cluster and leaf terminal samples), time (3,9 df for flower clusters and 10,30 df for leaf terminals), and the cultivar-by-time interaction (6,9 df for flower clusters and 20,30 df for leaf terminals) on the respective variables. The proportion of infected samples was transformed by arcsin square root before analysis. Standard errors were calculated from the appropriate residual mean-squared errors. This approach was used because there were insufficient numbers of plant subjects to analyze the variance-covariance structure; we therefore assumed homogeneity of variances and covariances.
Results
Mean relative BlScV concentration per g of infected leaf terminal and flower cluster in 2007 did not vary with cultivar (P > 0.28), but did vary with time (P < 0.0002), and the cultivar-by-time interaction was significant (P < 0.03 in each case). BlScV concentration in ’Dixi’ leaf tissue increased almost continuously, while concentration in ’Jersey’ increased until early June and then decreased and levelled out, and concentration in ’Rubel’ increased until mid-June and then declined (Fig. 2). Concentration in flower clusters increased rapidly to almost twice the maximum level in leaf terminals with the exception of ’Rubel’ in which concentration was relatively high initially and did not change with time (Fig. 2).
Figure 2
Mean relative BlScV concentration per g of highbush blueberry tissue versus time.
Flower bud or cluster arising from a single bud, open symbols (S.E. cultivar = 4.7, S.E. time = 3.3); leaf bud or leaves and stem arising from a single bud, closed symbols (S.E. cultivar = 4.5, S.E. time = 1.7); triangles, 'Dixi'; circles, 'Jersey'; diamonds, 'Rubel'.
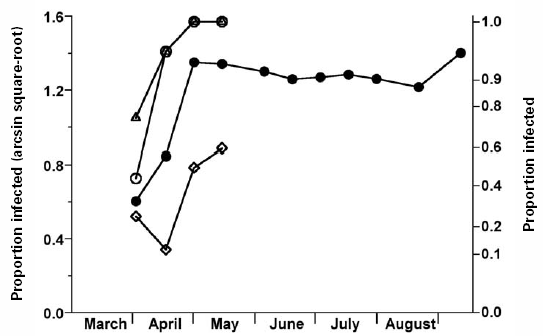
The proportion of infected leaf buds or terminals did not vary with cultivar (P = 0.21), but it varied with time (P < 0.0001). The proportion of infected flower buds or clusters varied with cultivar (P = 0.002) and time (P = 0.006); there was no cultivar-by-time interaction for either leaf or flower tissue (P > 0.34). The proportion of BlScV-infected samples increased through April to reach an asymptote of approximately 0.9 for leaf terminals, and to 1.0 for ’Dixi’ and ’Jersey’ flower clusters, but no asymptote was evident for ’Rubel’, the proportion of infected flower clusters increasing to 0.6 (Fig. 3).
Figure 3
Mean proportion of BlScV-infected highbush blueberry samples versus time (arcsin squared-root transformed data).
Flower bud or cluster arising from a single bud, open symbols (S.E. cultivar = 0.071, S.E. time = 0.16); triangles, 'Dixi'; circles, 'Jersey'; diamonds, 'Rubel'; leaf bud or leaves and stem arising from a single bud, solid circles (all cultivars combined; S.E. cultivar = 0.32, S.E. time = 0.086).
Mean relative BlScV concentration per infected leaf bud or terminal did not vary with cultivar (P = 0.23) but it did vary with time (P < 0.0001), and the cultivar-by-time interaction was significant (P = 0.001). Concentration in leaf terminals increased at the same rate in all three cultivars until early June and then diverged, with ’Dixi’ increasing most rapidly, ’Jersey’ less rapidly, and ’Rubel’ reaching an asymptote (Fig. 4). Concentration per flower bud or cluster did not vary with cultivar (P = 0.11) but it did vary with time (P < 0.0001), and the cultivar-by-time interaction was significant (P = 0.005); final concentration in ’Rubel’ was lower than in ’Dixi’ and ’Jersey’ (Fig. 4). Concentration per flower cluster did not increase to the level observed in leaf terminals because final flower cluster weight was smaller than leaf terminal weight later in the season: mean leaf terminal weight increased from 0.03 ± 0.001 to 5.8 ± 0.7 g whereas flower cluster weight increased from 0.07 ± 0.03 to 0.5 ± 0.02 g.
Figure 4
Mean relative BlScV concentration per highbush blueberry sample versus time.
Flower bud or cluster arising from a single bud, open symbols (S.E. cultivar = 1.4, S.E. time = 1.3); leaf bud or leaves and stem arising from a single bud, closed symbols (S.E. cultivar = 22.9, S.E. time = 8.8); triangles, 'Dixi'; circles, 'Jersey'; diamonds, 'Rubel'.
Discussion
The results have implications for sampling. BlScV surveys generally involve collecting mature leaves from a plant or group of plants. However, surveys could be conducted in early May using flower clusters to take advantage of their high virus concentration relative to that of leaf tissue collected later in the season (Fig. 2). If leaf tissue is sampled, our results suggest that studies of temporal variation in BlScV concentration are needed for each of the principal cultivars in a production area to determine optimal timing. The results of Dal Zotto et al. (1999), Torrance and Dolby (1984) and Varveri et al. (1997) corroborate this view for different virus-host plant systems. Similar cultivar-related considerations will undoubtedly affect aphid transmission studies and collection of plant tissue for virus purification.
BlScV concentration varied throughout the season (Fig. 2), and temporal patterns were different among the cultivars ’Dixi’, ’Jersey’, and ’Rubel’. However, the seasonal change in virus concentration was small compared with changes in numbers of a known BlScV vector, E. fimbriata, which colonizes highbush blueberry in the Pacific Northwest: Raworth (2004) found that populations of this aphid increase more than threefold during the spring. If virus concentrations in other cultivars show a seasonal variation comparable to that found here, then in general, one would expect E. fimbriata populations, rather than BlScV concentration, to be the more important factor driving virus transmission, and this should result in transmissions during June and July when E. fimbriata populations peak (cf. Raworth 2004). This prediction is supported by Raworth et al. (2008); 7 out of 938 trap plants placed in two BlScV-positive blueberry fields for 2-wk periods during 2001-2003 became infected in June and July while one became infected in May, and no other infections were detected. This result also concurs with Bristow et al. (2000). However, the effect of migrant aphids in this system is unknown. Raworth et al. (2006) found more than 80 aphid species migrating in blueberry fields; further work is needed to determine the vector status of a selection of some of these species. Until that work is done, the potential effect of the colonizing aphid E. fimbriata on BlScV transmission provides a good rationale for chemically controlling this species; not in June, when aphid numbers are typically high, but early in the season, after egg hatch and before exponential population increase (Raworth and Schade 2006) and production of alatae in May (Raworth 2004).
Appendices
Acknowledgements
We thank D. Schade for assistance in collecting samples, P. Ellis and G. de Villiers (Phyto Diagnostics Co. Ltd.) for DAS-ELISA tests, a blueberry grower for access to his field, Lisa Wegener for information about BlScV-infected plants, M. Sweeney for grower contacts, and T. Forge for useful comments on an earlier draft. Use of trade names or trademarks does not imply endorsement of the companies or products named nor criticism of similar ones not named. Pacific Agri-Food Research Centre contribution # 771.
References
- Bachand, G.D., and J.D. Castello. 1998. Seasonal pattern of Tomato mosaic tobamovirus infection and concentration in red spruce seedlings. Appl. Environ. Microbiol. 64 : 1436-1441.
- Bristow, P.R., R.R. Martin, and G.E. Windom. 2000. Transmission, field spread, cultivar response, and impact on yield in highbush blueberry infected with Blueberry scorch virus. Phytopathology 90 : 474-479.
- British Columbia Ministry of Agriculture and Lands. 2007. Berry Production Guide for Commercial Growers. Lower Mainland Horticulture Improvement Association, Abbotsford, BC, Canada.
- Cavileer, T.D., B.T. Halpern, D.M. Lawrence, E.V. Podleckis, R.R. Martin, and B.I. Hillman. 1994. Nucleotide sequence of the carlavirus associated with blueberry scorch and similar diseases. J. Gen. Virol. 75 : 711-720.
- Ciuffo, M., D. Pettiti, S. Gallo, V. Masenga, and M. Turina. 2005. First report of Blueberry scorch virus in Europe. Plant Pathol. 54 : 565.
- Clark, M.F., and A.N. Adams. 1977. Characteristics of the microplate method of enzyme-linked immunosorbent assay (ELISA) for the detection of plant viruses. J. Gen. Virol. 34 : 475-483.
- Dal Zotto, A., S.F. Nome, J.A. Di Rienzo, and D.M. Docampo. 1999. Fluctuations of Prunus necrotic ringspot virus (PNRSV) at various phenological stages in peach cultivars. Plant Dis. 83 : 1055-1057.
- Dovas, C.I., A.P. Mamolos, and N.I. Katis. 2002. Fluctuations in concentration of two potyviruses in garlic during the growing period and sampling conditions for reliable detection by ELISA. Ann. Appl. Biol. 140 : 21-28.
- Lowery, D.T., M.G. Bernardy, R.M. Deyoung, and C.J. French. 2008. Identification of new aphid vector species of Blueberry scorch virus. J. Entomol. Soc. B.C. 105 : 27-33.
- MacDonald, S.G., and R.R. Martin. 1990. Survey of highbush blueberries for scorch viruses. Can. Plant Dis. Surv. 70 : 96.
- Martin, R.R., and P.R. Bristow. 1988. A Carlavirus associated with blueberry scorch disease. Phytopathology 78 : 1636-1640.
- Pataky, J.K., C.C. Block, P.M. Michener, L.M. Shepherd, D.C. McGee, and D.G. White. 2004. Ability of an ELISA-based seed health test to detect Erwinia stewartii in maize seed treated with fungicides and insecticides. Plant Dis. 88 : 633-640.
- Polák, J. 1995. Reliability of detection and relative concentration of Plum pox virus determined by ELISA in an infected peach tree during the vegetative period. Z. Pflanzenkr. Pflanzenschutz. 102 : 16-22.
- Polák, J. 1998. Relative concentration of Plum pox virus in leaves and flowers of some Prunus species and cultivars. Acta Virol. 42 : 264-267.
- Raworth, D.A. 2004. Ecology and management of Ericaphis fimbriata (Hemiptera: Aphididae) in relation to the potential for spread of Blueberry scorch virus. Can. Entomol. 136 : 711-718.
- Raworth, D.A., and D. Schade. 2006. Life history parameters and population dynamics of Ericaphis fimbriata (Hemiptera: Aphididae) on blueberry, Vaccinium corymbosum. Can. Entomol. 138 : 205-217.
- Raworth, D.A., C.-K. Chan, R.G. Foottit, and E. Maw. 2006. Spatial and temporal distribution of winged aphids (Hemiptera: Aphididae) frequenting blueberry fields in southwestern British Columbia and implications for the spread of Blueberry scorch virus. Can. Entomol. 138 : 104-113.
- Raworth, D.A., C.J. French, D.T. Lowery, M.G. Bernardy, M. Bouthillier, S. Mathur, C.-K. Chan, R.G. Foottit, E. Maw, L.A. Wegener, and M. Sweeney. 2008. Temporal trends in the transmission of Blueberry scorch virus in British Columbia, Canada. Can. J. Plant Pathol. 30 : 345-350.
- Remaudière, G., and M. Remaudière. 1997. Catalogue of the world’s Aphididae. INRA, Paris, France. 473 p.
- Sutula, C.L., J.M. Gillett, S.M. Morrissey, and D.C. Ramsdell. 1986. Interpreting ELISA data and establishing the positive-negative threshold. Plant Dis. 70 : 722-726.
- Torrance, L., and C.A. Dolby. 1984. Sampling conditions for reliable routine detection by enzyme-linked immunosorbent assay of three ilarviruses in fruit trees. Ann. Appl. Biol. 104 : 267-276.
- Varveri, C., R. Holeva, and F.P. Bem. 1997. Effect of sampling time and plant part on the detection of two viruses in apricot and one in almond by ELISA. Ann. Inst. Phytopathol. Benaki 18 : 25-33.
- Wegener, L.A., R.R. Martin, M.G. Bernardy, L. MacDonald, and Z.K. Punja. 2006. Epidemiology and identification of strains of Blueberry scorch virus on highbush blueberry in British Columbia, Canada. Can. J. Plant Pathol. 28 : 250-262.
List of figures
Figure 1
Serial dilution data for three samples (circles, crossed circles, dark circles), with regressions (A), and the relationship used to convert DAS-ELISA OD readings to relative virus concentration (B).
Figure 2
Mean relative BlScV concentration per g of highbush blueberry tissue versus time.
Figure 3
Mean proportion of BlScV-infected highbush blueberry samples versus time (arcsin squared-root transformed data).
Figure 4
Mean relative BlScV concentration per highbush blueberry sample versus time.