Abstracts
Abstract
Heterobasidion irregulare is the scientific name for the North American fungal species that was previously known as H. annosum (P-type) and Fomes annosus. In eastern Canada, the pathogen is found mainly in red pine plantations in southern Ontario and Quebec, where it causes tree mortality. There is no registered control method currently available for this disease in Canada. Phlebiopsis gigantea is a saprophytic basidiomycete successfully used and registered as a biological control agent in several European countries. In order to register a control product in Canada, its efficacy must be demonstrated under field conditions. Trials were performed with two Canadian isolates of P. gigantea in four red pine plantations in Ontario. The mean diameters of treated stumps ranged from 29 to 35 cm. After 2 mo, all 238 stumps treated were free of disease, while 12% of the 120 untreated stumps were colonized by the pathogen. The two formulations without P. gigantea did not prevent the colonization of the stumps by either P. gigantea or H. irregulare. These results show that the two Canadian isolates of P. gigantea can prevent colonization of red pine stumps by H. irregulare and provide support for the registration of P. gigantea as a biocontrol agent in eastern Canada.
Keywords:
- Biological control,
- efficacy trial,
- Heterobasidion irregulare,
- Phlebiopsis gigantea,
- Pinus resinosa
Résumé
Heterobasidion irregulare est le nom scientifique de l’espèce fongique nord-américaine connue auparavant sous le nom de H. annosum (forme P) et Fomes annosus. Dans l’Est canadien, ce champignon pathogène touche principalement les plantations de pins rouges du sud de l’Ontario et du Québec, où il cause la mort des arbres. Au Canada, aucun produit de contrôle homologué n’est disponible pour lutter contre cette maladie. Phlebiopsis gigantea est un basidiomycète saprophyte utilisé avec succès et homologué comme agent de lutte biologique dans plusieurs pays d’Europe. Pour homologuer un produit semblable au Canada, son efficacité doit être démontrée en forêt. Des essais ont été effectués dans quatre plantations de pins rouges en Ontario avec deux isolats canadiens de P. gigantea. Le diamètre moyen des souches traitées variait de 29 à 35 cm. Après deux mois, les 238 souches traitées n’avaient pas été colonisées par H. irregulare alors que 12 % des 120 souches non traitées l’étaient. Les deux formulations sans P. gigantea n’ont pas prévenu la colonisation des souches ni par P. gigantea, ni par H. irregulare. Ces résultats montrent que les deux isolats canadiens de P. gigantea peuvent prévenir la colonisation des souches de pin rouge par H. irregulare et ils viennent supporter l’homologation de P. gigantea comme agent de lutte biologique dans l’Est du Canada.
Mots-clés :
- Lutte biologique,
- Heterobasidion irregulare,
- Phlebiopsis gigantea,
- Pinus resinosa,
- test d’efficacité
Article body
Introduction
Heterobasidion irregulare (Underw.) Garbel. & Otrosina is the new scientific name for the North American species that was known for a long time as Fomes annosus (Fr.: Fr.) Cooke or, more recently, as the P-type of Heterobasidion annosum (Fr.: Fr.) Bref. (Otrosina and Garbelotto 2010). Heterobasidion irregulare is a basidiomycete with fruiting bodies produced on stumps or at the base of infected pines. It causes a root disease capable of killing seedlings and large trees. The primary mode of infection of this aggressive pathogen is through the release of basidiospores from basidiocarps, which are produced just below the duff layer. Successful infections can only occur if the basidiospores are deposited on freshly cut stump surfaces. Once H. irregulare is established in a pine stump, it can use grafts between infected and non-infected roots to further its spread within a plantation. Depending on pine species and geographical locations, H. annosum in Europe (Woodward et al. 1998), which is very similar to H. irregulare, can survive in stumps for 10 to 46 yr (Redfern and Stenlid 1998), even up to 62 yr (Greig and Pratt 1976), thus making it a chronic infectious agent through several silvicultural treatments.
In Canada, Jorgensen (1956) was the first to report the presence of H. irregulare on red pine (Pinus resinosa Ait.) at St. Williams, in Ontario. That pine stand had been thinned for the first time in 1929. By 1968, the pathogen had spread further north, near Ottawa, Ontario (Sippell et al. 1968). The disease has since been found in Quebec (Laflamme and Blais 1995) and new infection centres have become established in several different regions of that province. In eastern Canada, the pathogen is found mainly in red pine plantations in southern Ontario and Quebec where it kills pines. The disease is progressing northward. The northernmost extension of the disease reported in 2007 is located at Saint-Jean-de-Matha, Quebec (46°14'N; 73°32'W), which is 800 km north of St. Williams. There are natural stands of jack pine (Pinus banksiana Lamb.) less than 50 km away from Saint-Jean-de-Matha and the disease could have an impact on that important tree species.
Regenerating conifers in the mortality pockets can become infected and mortality of Pinus strobus L. and Abies balsamea (L.) Mill. has been observed (Laflamme and Blais 1995; Dumas, unpublished data). Most infections occur when basidiospores infect freshly cut stumps after a stand is thinned (Rishbeth 1951). Once the fungus is established in the plantation, it will spread to adjoining healthy trees through root grafts. Red pine plantations occupy a very large area of the productive forested land in southern Ontario and Quebec. Periodic thinning of the stand is a silvicultural prescription in order to increase stand quality and achieve the end product desired, be it telephone poles, saw logs or pulp. However, thinning makes these stands very susceptible to H. irregulare. In a field trial, H. irregulare was able to colonize freshly cut stumps of jack pine (Dumas and Laflamme, submitted), and it is probable that all pine species, including P. strobus and P. sylvestris L., can be affected by this disease.
In Ontario, borax was used beginning in the early 1970s, and a few years later in Quebec, as a prophylactic treatment on freshly cut stumps to reduce the possibility of new infections during stand thinning (Myren and Punter 1972). In December 2001, however, it was removed as a registered product in Canada and there is currently no control method available for this disease in Canada. The successful viability of existing pine plantations and the reestablishment of new ones would be facilitated by the availability of an environmentally safe and effective method to manage this disease.
Phlebiopsis gigantea (Fr.:Fr.) Jülich. is a saprophytic basidiomycete that is widely distributed throughout the conifer forests of the Northern Hemisphere (Holdenrieder and Greig 1998). It is an indigenous organism beneficial to red pine stands because it plays an important role in the decay and breakdown of moribund and dead wood and poses no risk to living trees or agricultural crops. It has been successfully used and is currently registered as a biological control agent in Europe to manage spruce and pine plantations colonized respectively by Heterobasidion parviporum Niemelä & Korhonen (Fr.) and H. annosum. Development of this biological control method could lead to its use as a commercial product in Canada and the northern United States. When applied to freshly cut stumps, it competes directly with the pathogen for the wood resource (Pratt et al. 1999, 2000). In Europe, three different formulations are, or have been, available: PG Suspension (PGS) in the UK; PG IBL in Poland; and Rotstop® in Scandinavia (Pratt et al. 2000). Rotstop® is registered and commercially available (Pratt et al. 2000), but PGS and PG IBL may not meet the requirements of the European community. In the United States, P. gigantea was commercially available until 1995, but the Environmental Protection Agency notified the USDA Forest Service that since it was biological, pesticide registration would be needed and production ceased (Cram 2012).
Past research indicates that European isolates of P. gigantea should not be transferred to North America due to differences in their genetic compositions (Vainio and Hantula 2000). However, new data tend to show that the European and Canadian strains of P. gigantea can be regarded as representing the same biological species (Grillo et al. 2005), but this is not an issue since we have efficacious isolates of P.gigantea from Canada (Bussières et al. 1996). However, efficacy trial under specific ecological conditions is still a requirement for registration. The use of Canadian isolates would make it possible to avoid the introduction of non-indigenous strains into the gene pool. Application methods have been developed for commercial equipment, which consists of a special bar or a spray nozzle attached to the cutting heads of harvesters. The efficacy of this equipment has been demonstrated in Europe (Thor and Stenlid 1998; Pratt and Thor 2001) and could be quickly adapted for use in Canadian pine stands.
In order to register a biological control product in Canada, its efficacy must be demonstrated under field conditions. The objective of this study was first to determine the efficacy of two isolates of P. gigantea; if one of these isolates is lost or degenerating, the other one could be used without having to repeat the efficacy trial. The second objective was to see the effect of formulations without P. gigantea on the colonization of red pine stumps by wild P. gigantea and H. irregulare.
Materials and methods
Field trials
The efficacy trials of two P. gigantea isolates for the control of H. irregulare were performed in four red pine plantations in southern Ontario. Annosum root disease was present in the plantations or in plantations nearby. The four plantations were located in York County (44°32'50.1''N; 79°19'58.1''W), Dufferin County (44°12'46.4''N; 80°02'54.2''W), Breedon, Simcoe County (44°12'46.4''N; 79°33'03.5''W), and Stoney, Simcoe County (44°35'03.7''N; 79°49'25.8”W).
Four experiments were conducted in parallel using fresh stumps in the four red pine plantations:
Pg-92-104 (Verdera), a P. gigantea isolate formulated by Verdera Inc., Finland. This isolate came from a red pine log sampled in 1992 at Harrington, QC, and is part of the P. gigantea collection at Université Laval. The formulation used is the same as the one used in Rotstop®; it is a dry powder diluted at the rate of 1 g L-1 of water to give a concentration of 1 x 106–107 cfu L-1;
Pg-GLFC-ALS, a P. gigantea isolate formulated by M. Dumas at the Great Lakes Forestry Centre (GLFC), with the addition of 3% ALS (ammonium lignosulfonate). This isolate came from a red pine log sampled in Sault Ste. Marie, ON. The Pg-GLFC-ALS solution was prepared at a concentration of 1 x 106–107 cfu L-1;
Vedera formulation, which is a powder without the P. gigantea used in Rotstop®, formulated by Verdera Inc.;
GLFC-ALS formulation, a water solution of 3% ALS without P. gigantea, prepared by M. Dumas.
Control A and Control B are stumps left untreated to evaluate the inoculum rate of P. gigantea and H. irregulare in the stands after 2 mo and 1 yr, respectively.
Experiments were conducted with 40-50 cm high stumps during commercial thinning operations using harvesters (Fig. 1). A total of 720 trees were harvested in the last week of August 2005 in the York and Dufferin plantations, and in the second week of September 2005 in the Breedon and Stoney plantations. In each of the four red pine plantations, 180 red pine stumps were identified by a number and 30 were randomly treated with one of the two P. gigantea isolates, one of the two formulations, or were left untreated for the two control groups. Treatments were done within 5 min after felling and solutions were applied using manual sprayers to completely cover the stump surface (Fig. 2). In mid-November 2005, 2 mo after inoculation, 10-cm discs were cut from the top of the stumps. The 600 discs representing samples from the four trials and control A were harvested, placed in separate paper bags and stored in a cold room at –2°C until being processed in the laboratory within 2 mo after collection. In the fall of 2006, the remaining 120 discs representing Control B were collected and processed the same way.
Figure 1
A tree harvester used in this trial for thinning red pines at the York site.
Figure 2
Stump treatments with two P. gigantea formulations and two formulations without P. gigantea were done manually with hand sprayers in four Ontario red pine plantations.
Laboratory processing
Discs were removed from the cold room 16 h before the isolation procedures. The diameter of each wood disc was measured before it was cut like a pie into eight wedges on a band saw. From each wedge, a thin layer of wood was removed with a sterile scalpel before removal of a wood chip. The wood chips were taken from the inside section at a one-third and two-third distance between the bark and the heartwood using a sterile scalpel, for a total 16 chips per wood disc. Four wood chips were placed on an isolation medium and incubated at 25°C. The isolation culture medium (30 g L-1 malt extract, 15 g L-1 agar) was prepared in 490 mL volumes and autoclaved for 20 min at 121°C. The medium was cooled to 60°C, and we added 10 mL of a filter sterilized aqueous solution containing 50 mg neomycin sulphate, 50 mg chlorotetracycline, 500 mg streptomycin sulfate and 25 µg methyl-1-benzamidazole carbamate phosphate (Lignasan®) as used by Wiensczyk et al. (1996) and modified slightly by the first author. This medium inhibits the growth of moulds but not that of basidiomycetes, allowing for easier and more accurate detection of P. gigantea and H. irregulare. Cultures were maintained on 3% malt extract–1.5% agar (MA) medium.
The identification of the P. gigantea strains (Pg-92-104 and Pg-GLFC) recovered from wood discs on stumps treated with P. gigantea was confirmed by vegetative compatibility crossing (Roy et al. 1997).
Results
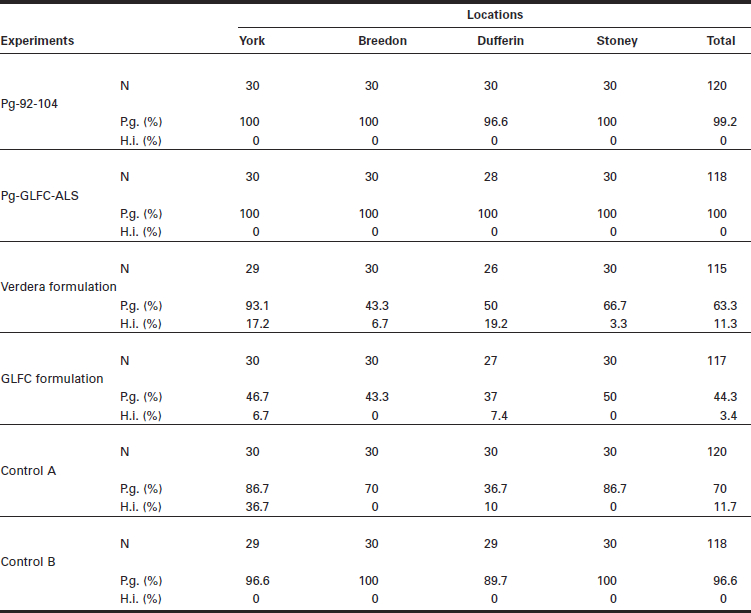
The diameters of treated stumps in the four sites averaged 32.5 cm (Table 1); it is representative of the size of red pines harvested in second thinning. Two months after inoculation, 100% of the stumps treated with the two P. gigantea isolates (Pg-92-104 and Pg-GLFC) were colonized, and no infection by H. irregulare was detected (Table 2). The identification of the P. gigantea strains on these treated stumps was confirmed by vegetative compatibility crossing and we recovered 100% of our two isolates in each of the two experiments conducted with Pg-92-104 and Pg-GLFC-ALS.
Table 1
Mean diameter (cm) at stump level for samples collected at four southern Ontario experimental sites.
Table 2
Infection rates (%) of Phlebiopsis gigantea (P.g.) and Heterobasidion irregulare (H.i.) 2 mo after stump treatments with Pg-92-104, Pg-GLFC-ALS, Verdera and GLFC formulations without P. gigantea, no treatment (Control A) samples harvested after 2 mo, and no treatment samples (Control B) collected after 1 yr. Experiments were done in red pine plantations at the four Ontario sites of York, Breedon, Dufferin and Stoney.
Stumps sprayed with the two formulations without P. gigantea did not prevent infections by wild P. gigantea and H. irregulare; on stumps treated with the Verdera and GLFC formulations, wild P. gigantea was present on average on 63.3% and 44.3% of the stumps, respectively, while H. irregulare was present on 11.3% and 3.4% of the stumps (Table 2). The infection rates of wild type P. gigantea strains isolated from untreated stumps ranged from 70% on wood discs collected after 2 mo to 96.6% on samples collected after 1 yr (Table 2). While 11.7% of untreated stumps (Control A) sampled after 2 mo tested positive for H. irregulare, the pathogen was not observed on stumps collected 1 yr after treatment (Control B).
Discussion
The two Canadian isolates of P. gigantea (Pg-92-104 and Pg-GLFC-ALS) used in this efficacy test proved to be excellent biological agents by colonizing all treated red pine stumps and preventing 100% of them from invasion by H. irregulare. An earlier efficacy trial done with five different P. gigantea isolates, including the same Pg-92-104 isolate but formulated by Université Laval, gave the same results (Bussières et al. 1996). Moreover, that test was done during three different periods of the growing season, in May, July and September, on three different sites, and the Pg-92-104 isolate colonized all stumps with the exception of one location in July, where 60% of the stumps were colonized. It seems that dry conditions and high temperatures in July may have had a negative effect on colonization by P. gigantea (Bussières et al. 1996). This may be alleviated by adding ammonium lignosulfonate to the formulation because that compound has a beneficial effect under those conditions (Dumas 2011).
The size of red pines used for this test, with an average diameter of 32.5 cm, is representative of trees harvested during second thinning. The relatively large surface of the stumps in this trial provided a good substrate for a higher probability of colonization by the pathogen: after 2 mo, 11.7% of the control stumps were infected by H. irregulare.
The treatment was done manually with hand sprayers (Fig. 2); an automated sprayer of tree harvester (Fig. 1) could give similar results once the calibration is done properly. The carrier solutions by themselves did not prevent colonization of the stumps by either P. gigantea or H. irregulare, confirming their benign effect on both P. gigantea and H. irregulare.
Phlebiopsis gigantea is naturally very common in the ecological regions where the efficacy trials took place. This saprophyte colonized 70% of untreated red pine stumps. In spite of this high occurrence of natural P. gigantea, we cannot rely on this natural inoculum for disease control. In a similar test conducted in the province of Quebec, the rate of colonization of untreated stumps was quite variable, ranging from 40 to 100%, and much lower when stumps were treated with borax or urea (Bussières et al. 1996). Also, both wild P. gigantea and H. irregulare can grow on the same stumps, but P. gigantea may only occupy a small portion of the stump surface. Spraying a suspension of P. gigantea spores on stump surfaces results in a more complete protection of the potential infection court.
Inoculum rates of P. gigantea in the four experimental sites were relatively high; the average rates on stumps treated with the two formulations alone or on untreated stumps was 63.3%, 44.3 %, 70% and 96.6%, respectively. On the other hand, inoculum rates of H. irregulare were variable from site to site. In York, up to 36.7% of untreated stumps collected after 2 mo were colonized by the pathogens. In this area, we had tree mortality caused by H. irregulare within the experimental sites; this may explain the result. At the Dufferin site, we recorded up to 19.2%, which is quite high. It is known that H. irregulare needs conducive conditions when the spores reach the stump, while spores from P. gigantea can colonize a stump over a longer time period (Holdenreider and Greig 1998). The inoculum rate of H. irregulare was low at the two other sites (Breedon and Stoney), but still present as we recorded 6.7% and 3.3% in one of the four trials with formulation only or untreated.
The results of this efficacy trial with Canadian isolates of P. gigantea provide support for the registration and commercialization of P. gigantea in eastern North America as an effective biological control product to prevent infection of red pine stumps by H. irregulare.
Appendices
Acknowledgements
The authors are indebted to Nick Boyonoski, Gilles Bélanger, Martine Blais and Julie Dubé (Canadian Forest Service) for their excellent assistance. We are most grateful to James Lane and Ian Buchanan of the Regional Municipality of York; Caroline Mach of Dufferin County; and Graeme Davies and Bob Hutchinson of Simcoe County for their help with the research areas. We thank Pamela Cheers from the Laurentian Forestry Centre for editing the text and to external reviewers for their corrections and suggestions.
References
- Bussières, G., A. Dansereau, M. Dessureault, G. Roy, G. Laflamme, and R. Blais. 1996. Lutte contre la maladie du rond dans l’ouest du Québec : Projet no 4023. Essais, expérimentations et transfert technologique en foresterie. Ressources naturelles Canada, Service canadien des forêts, Centre de foresterie des Laurentides, Sainte-Foy, QC.
- Cram, M. 2012.Phlebiopsis (=Phanerochaete, =Peniophora, =Phlebia, =Peniophora) gigantea (Basidiomycetes: Corticiaceae) in A. Shelton (ed.), Biological control. A guide to natural enemies in North America. Available on-line at http://www.biocontrol.entomology.cornell.edu//pathogens/phlebiopsis.html. Cornell University, Ithaca, NY, USA.
- Dumas, M.T. 2011. Stimulatory effect of ammonium lignosulfonate on germination and growth of Phlebiopsis gigantea spores. For. Pathol. 41 : 189-192.
- Dumas M.T., and G. Laflamme.Heterobasidion irregulare in eastern Canada and its potential to invade Pinus banksiana stands. Can. Plant Dis. Surv. (submitted).
- Greig, B.J.W., and J.E. Pratt. 1976. Some observations on the longevity of Fomes annosus in conifer stumps. Eur. J. For. Pathol. 6 : 250-253.
- Grillo, R., J. Hantula, and K. Korhonen. 2005. Interfertility between North American and European strains of Phlebiopsis gigantea. For. Pathol. 35 : 173-182.
- Holdenrieder, O., and B.J.W. Greig. 1998. Biological methods of control. Pages 235-258 in S. Woodward et al. (eds.), Heterobasidion annosum: biology, ecology, impact and control. CAB International, Wallingford, UK.
- Jorgensen, E. 1956.Fomes annosus (Fr.) Cke. on red pine in Ontario. For. Chron. 32 : 87-88.
- Laflamme, G., and R. Blais. 1995. Détection du Heterobasidion annosum au Québec. Phytoprotection 76 : 39-43.
- Myren, D.T., and D. Punter. 1972. An evaluation of six methods for protecting pine stump tops from infection by Fomes annosus in Ontario. Information Report O-X-164. Natural Resources Canada, Canadian Forest Service, Great Lakes Forestry Centre, Sault Ste. Marie, ON.
- Otrosina, W.J., and M. Garbelotto. 2010.Heterobasidion occidentale sp. nov. and Heterobasidion irregulare nom. nov.: A disposition of North American Heterobasidion biological species. Fung. Biol. 114 : 16-25.
- Pratt, J.E., J.N. Gibbs, and J.F. Webber. 1999. Registration of Phlebiopsis gigantea as a forest biocontrol agent in the UK: Recent experience. Biocontrol Sci. Technol. 9 : 113-118.
- Pratt, J.E., M. Niemi, and Z.H. Sierota. 2000. Comparison of three products based on Phlebiopsis gigantea for the control of Heterobasidion annosum in Europe. Biocontrol Sci. Technol. 10 : 467-477.
- Pratt, J.E., and M. Thor. 2001. Improving mechanized stump protection against Fomes root rot in Europe. J. For. 95 : 119-127.
- Redfern, D.B., and J. Stenlid. 1998. Spore dispersal and infection. Pages 105-124 in S. Woodward et al. (eds.), Heterobasidion annosum: biology, ecology, impact and control. CAB International, Wallingford, UK.
- Rishbeth, J. 1951. Observations on the biology of Fomes annosus with particular reference to East Anglian pine plantations. II. Spore production, stump infection, and saprophytic activity in stumps. Ann. Bot. 15 : 1-22.
- Roy, G., M. Cormier, M. Dessureault, and R.C. Hamelin. 1997. Comparison of RAPD technique and somatic incompatibility tests for the identification of Phlebiopsis gigantea strains. Can. J. Bot. 75 : 2097-2104.
- Sippell, W.L., A.H. Rose and H.L. Gross. 1968. Ontario Region: Important Forest Diseases. Pages 66-76 in Annual Report of the Forest Insect and Disease Survey, Department of Fisheries and Forestry, Ottawa, ON.
- Thor, M., and J. Stenlid. 1998.Heterobasidion annosum infection following mechanized first thinning and stump treatment in Picea abies. Pages 397-407 in C. Delatour et al. (eds.), Proceedings of the 9th International Conference on Root and Butt Rots, September 1-7, 1997, Carcans-Maubuisson, France. INRA, Paris, France.
- Vainio, E.J., and J. Hantula. 2000. Genetic differentiation between European and North American populations of Phlebiopsis gigantea. Mycologia 92 : 436-446.
- Wiensczyk, A.M., M.T. Dumas, and R.N. Irwin. 1996. Effect of Northwestern Ontario Forest Ecosystem Classification treatment units on the infection levels of Armillaria in black spruce plantations. Can. J. For. Res. 26 : 1248-1255.
- Woodward, S., J. Stenlid, R. Karjalainen, and A. Hüttermann (eds.). 1998.Heterobasidion annosum: biology, ecology, impact and control. CAB International, Wallingford, UK.
List of figures
Figure 1
A tree harvester used in this trial for thinning red pines at the York site.
Figure 2
Stump treatments with two P. gigantea formulations and two formulations without P. gigantea were done manually with hand sprayers in four Ontario red pine plantations.
List of tables
Table 1
Mean diameter (cm) at stump level for samples collected at four southern Ontario experimental sites.
Table 2
Infection rates (%) of Phlebiopsis gigantea (P.g.) and Heterobasidion irregulare (H.i.) 2 mo after stump treatments with Pg-92-104, Pg-GLFC-ALS, Verdera and GLFC formulations without P. gigantea, no treatment (Control A) samples harvested after 2 mo, and no treatment samples (Control B) collected after 1 yr. Experiments were done in red pine plantations at the four Ontario sites of York, Breedon, Dufferin and Stoney.





 10.7202/706083ar
10.7202/706083ar