Résumés
Abstract
Abutilon theophrasti (Malvaceae) is a troublesome annual weed in many maize and soybean cropping systems of Canada and the United States. Seeds of A. theophrasti exhibit physical dormancy. Differences in the growing environment of parent plants may influence the germinability of seeds and vigour of seedlings produced by this species because of variation in resource allocation to seed development. Thus, the germinability of seeds and subsequent seedling vigour were examined for A. theophrasti plants grown in monoculture at a density of 4.2 plants m-2 under varying natural photoperiods in central New York State. Treatments were established by transplanting A. theophrasti seedlings on three dates: 15 May, 4 June, and 30 June 2000, which correspond to peak photoperiods of 15, 14, and 13 hours, respectively. Seeds produced under the shorter photoperiod (13 h) weighed, on average, 1.5 mg less than seeds produced under the longer photoperiod (15 h). Contrary to expectations, seeds of A. theophrasti that matured under shorter photoperiods had lower germinability (80%) than seeds produced under longer photoperiods (98%). Early radicle growth, a measure of seedling vigour, did not differ between the photoperiod treatments. Environmental conditions other than photoperiod (i.e. water availability) prevailing during the 2000-growing season may have influenced seed coat thickness and consequently affected the germinability of seeds.
Résumé
L’Abutilon theophrasti (Malvaceae) est une mauvaise herbe annuelle qui gêne la production du maïs et du soja dans plusieurs systèmes de culture du Canada et des États-Unis. Les graines de l’A. theophrasti possèdent une dormance physique. Des différences de l’environnement dans lequel croissent les plantes mères peuvent influencer la germination des graines et la vitalité des plantules de cette espèce à cause de variations dans l’affectation des ressources avant que les graines soient pleinement développées. Ainsi, la germination des graines et la vitalité des plantules qui en sont issues ont été étudiées pour l’A. theophrasti en monoculture à une densité de 4,2 plantes m-2 sous diverses photopériodes naturelles du centre de l’État de New York. Les traitements ont débuté par la plantation de plantules de l’A. theophrasti à trois dates, 15 mai, 6 juin et 30 juin 2000, qui correspondent respectivement à des photopériodes maximales de 15, 14 et 13 heures. Les graines produites avec la photopériode la plus courte (13 h) pesaient en moyenne 1,5 mg de moins que les graines produites avec les plus longues photopériodes (15 h). Contrairement à ce qui était prévu, les graines de l’A. theophrasti qui se sont formées lors des photopériodes les plus courtes avaient une germination plus faible (80 %) que les graines formées lors des photopériodes les plus longues (98 %). La croissance précoce de la radicule, une mesure de la vitalité des plantules, est restée la même pour les différentes photopériodes. Les conditions environnementales autres que la photopériode (c.-à-d. la disponibilité en eau) qui régnaient au cours de la saison de croissance de 2000 peuvent avoir influencé l’épaisseur du tégument et avoir ainsi eu un effet sur la germination des graines.
Corps de l’article
Introduction
The germinability of seeds is influenced by many factors occurring both pre- and post-dispersal from the parent plant. Much focus has been placed on the maternal environment (pre-dispersal) and how variations within this environment alter the growth and reproduction of maturing plants (Fenner 1991; Gutterman 2000). Factors such as photoperiod, light quality, and competition have been shown to be most important (Fenner 1991). The length of daily exposure to solar radiation (photoperiod) is clearly an important trigger for initiation of floral development in many plant species (Huang et al. 2000; Patterson 1995; Stirling et al. 2002). Sensitivity to photoperiod may delay growth and, in some cases, decrease the time required for flowering and seed set, but it is unclear how this may influence the germination of seeds produced that possess physical dormancy.
The interval between germination and floral initia-tion is variable and species dependent. A decrease or, alternatively, an increase in the period between ger-mination and floral initiation may have a major im-pact on reproductive output of individuals within a population. A shorter interval between germination and flowering may significantly reduce aboveground biomass accumulation and seed output. For example, Amaranthus retroflexus L. plants (a quantitative short-day species) grown under a long photoperiod (16 h) took longer to reach reproductive maturity than plants grown under a short photoperiod (8 h) (Huang et al. 2000). Plants subjected to the short-day treatment initiated flowering 27 d after germination while plants subjected to the long-day treatment required 50 d to begin flowering. Moreover, the number of seeds, leaves, and dry weight of shoots and inflorescences increased with increasing photoperiod. Similar results have been reported for other species including the perennial Zingiber mioga Roscoe (Stirling et al. 2002) and the annual Abutilon theophrasti Medic. (Patterson 1995) in which time to flowering, number of flowers, and aboveground biomass increased for individuals maturing under longer photoperiods.
Although different types of seed dormancy exist, these have generally been classified into five catego-ries: physiological, morphological, morphophysio-logical, physical, and combinational (physiological + physical) dormancy (Baskin and Baskin 1998, 2004). Numerous studies have examined the effect of photo-period on plant growth and germinability of seeds produced for quantitative short-day plants posses-sing physiological or morphological seed dormancy, and have generally reported that the proportion of dormant seeds increases when parent plants mature under a longer versus shorter photoperiod (Fenner 1991). However, few studies have investigated the effect of photoperiod in species whose seeds exhibit physical dormancy or hardseededness. In one such study, Evenari et al. (1966) reported that Ononis sicula Guss. plants maturing under a long photoperiod (20 h) produced seeds with thicker, more impermeable seed coats than plants maturing under a short pho-toperiod (8 h).
Abutilon theophrasti (medic.) is a quantitative short-day herbaceous annual that colonizes highly disturbed habitats such as agricultural fields and waste areas in eastern and central North America (Oliver 1979; Patterson 1995; Warwick and Black 1988). Although there have been previous studies on the effect of photoperiod on growth and reproduction in this species (Patterson 1995), no research to date has linked the effect of photoperiod to the ability of seeds produced to germinate. Time to flowering in this species is most rapid under short photoperiods (e.g. 11 h; 30 days after emergence (DAE)), although A. theophrasti does eventually flower under longer photoperiods (e.g. 15 h; 45 DAE). Under glasshouse conditions, plant height, fruit weight, internode length, and shoot weight of A. theophrasti plants were found to increase with increasing photoperiod (Patterson 1995). Oliver (1979) reported that A. theophrasti directly seeded into a soybean crop (Glycine max L. Merr.) in May in Arkansas, USA, flowered later and had greater vegetative growth than plants transplanted in July. However, the authors did not provide any data on seed output and germinability of seeds produced.
Most A. theophrasti seeds exhibit physical dormancy and as such cannot imbibe water unless the seed coat and/or chalazal opening are fractured (Horowitz and Taylorson 1984, 1985; LaCroix and Staniforth 1964; Warwick and Black 1988). Bello et al. (1995) reported that A. theophrasti plants grown at three monoculture densities in full sunlight from May to September in a 2-yr study in Ames, Iowa USA, produced seeds that were nearly 20% less likely to germinate (i.e. were more dormant) when subjected to optimal germination conditions compared with plants that were grown under artificial shade. Plants under shade also produced fewer capsules and seeds, and had significantly lower dry weight than non-shaded plants. This finding suggests that plants under shade may have had limited resources available to invest into reproduction including dormancy-related mechanisms, although Bello et al. (1995) were unsure whether the decreased dormancy of seeds produced from plants under shade was due to the production of fewer seeds with impermeable seed coats or to other dormancy mechanisms.
At present, it is unclear what effect photoperiod differences experienced by parental A. theophrasti plants in the field would have on the germinability of seeds produced and the vigour of seedlings originating from these seeds. Results from this study may be important for improving long-term management of A. theophrasti by improving our understanding of the germinability and subsequent seedling vigour of seeds produced by plants maturing late in the growing season in areas of poor crop establishment or along field edges. We hypothesize that A. theophrasti plants grown under long photoperiods (e.g. peak 15 h) will produce a greater proportion of dormant seeds, with these seeds giving rise to more vigourous seedlings than seeds produced under shorter photoperiods (e.g. peak 13 h). Hence, the objectives of this study were to determine the effect of varying natural photoperiod on: (1) the germinability of A. theophrasti seeds and (2) seedling vigour as measured by the initial rate of radicle growth.
Materials and methods
Initial seed source
In autumn 1999, mature seeds of A. theophrasti required to produce transplants for the field study during the 2000 growing season were randomly collected from plants growing in a maize (Zea mays L.) crop in Aurora, NY, USA (42°45’N, 76°39’W). Seeds of relatively equal weight (9.0 - 9.5 mg) were stored dry at 5°C in paper bags until they were needed in late April 2000.
Study site
The field trial was established in May 2000 at the Robert Musgrave Agronomy Research Farm of Cornell University in Aurora, NY. The study site was located on a lima silt loam with a 2.6% organic matter content, and a pH of 7.9. The previous crop grown on the site was glyphosate- ((N-phosphonomethyl)glycine) resistant soybean. In early May 2000, the soil was moldboard plowed, disked, and the non-selective herbicide glyphosate was applied at 0.9 kg a.i. ha-1 to control existing vegetation prior to planting.
Seedling source and field experiment
In late April 2000, seeds of A. theophrasti collected in autumn 1999 (see initial seed source) were germinated by placing in cheesecloth, soaking in boiling water for 10 s, and placing on filter paper moistened with distilled water in Petri dishes. Petri dishes were placed on a laboratory countertop at ambient temperature (ca. 22-23°C, 50-60% RH). Nearly all seeds germinated within 3 d. Individual germinated seeds were placed in peat pots (40 cm3) containing a 1:1 v:v mixture of peat moss and vermiculite and micro- and macronutrients. Pots were transferred to a glasshouse under a 14-h natural photoperiod and 30/15°C (± 3°C) day/night temperatures. On relatively warm (> 15°C) partly cloudy d, pots were moved outdoors to harden seedlings.
To assess the effect of different photoperiods (peak 13-15 h) on A. theophrasti capsule development and maturation, three transplant dates were used: 15 May, 4 June, and 30 June. The early and late dates correspond to typical (8 May) and late (23 June) sowing dates for maize in central NY State. A week prior to transplanting, fertilizer (10-20-20, N-P-K) was added to plots at a rate of 224 kg ha-1. For each of the transplant dates, seedlings were planted into the field at the 2- to 3-leaf stage. To minimize transplant shock, seedlings received about 200 mL of tap water at planting. Treatment plots measured 9 m x 2.28 m and comprised three rows of A. theophrasti seedlings with 25 seedlings planted per row. Seedlings within a row were planted 30 cm apart and rows were 76 cm apart, resulting in a planting density of 4.2 plants m-2. The experiment was set up as a randomized complete block design with four blocks resulting in four repli-cate plots for each of the three transplant dates. Plots were weeded regularly both by hand and with a hoe throughout the growing season to remove unwanted weeds. Starting in mid-August and ending in early October, mature capsules were collected randomly twice weekly from each of twenty A. theophrasti trans-plants from the middle row only. A. theophrasti cap-sules are fully mature when capsules turn from a deep green to a black colour and loose seeds within the capsules produce a rattling sound. During har-vesting, seed losses due to shattering of capsules were minimized by placing collection bags directly under the capsules prior to removal from plants. Harvested capsules were stored dry at ambient tem-perature (ca. 22-23°C, 50-60% RH) in the laboratory prior to seed separation. In early November, capsules were carefully opened by hand and seed separated from chaff using sieves. For each of the three trans-planting dates, all harvested seeds were combined and stored dry at 5°C for 4 mo in coin envelopes until the start of germination experiments in May 2001.
Soil moisture (volumetric water content) was measured using time domain reflectrometry (TDR). Soil moisture probes of 30 cm length were inserted into the soil at a 45° angle so as to measure the soil moisture in the top 20 cm of the soil. One probe was placed within each experimental plot. Measurements using TDR were verified in the lab by drying soil samples of known volume in an oven for 48 h at 105°C and determining volumetric water content.
Germination trial
The mean weight of 150 randomly selected seeds from each transplant date treatment was determined to the nearest mg. These seeds were then used in the germination trial. Seed germination experiments were conducted in a growth chamber set at 27/14°C day/night temperatures and a 14-h photoperiod. The photosynthetic photon flux density (PPFD) averaged 150 µmol m-2 s-1 within the chamber. Thirty seeds from each A. theophrasti transplant date treatment were placed in a 9-cm diam Petri dish on one layer of filter paper (Fisher Brand Size P2) and moistened with 5 mL of distilled water. Five replicates each of 30 seeds per transplant date treatment were used. Seed ger-mination was monitored daily for 14 d. A seed was considered to have germinated when the radicle was at least 1 mm long. Germinated seeds were imme-diately removed from Petri dishes and used in the radicle elongation experiment described below. The viability of seeds failing to germinate by the end of the 14-d experimental period was assessed by a pressure test where light pressure was applied to the seeds with tweezers. Seeds succumbing to the light pressure or that were heavily colonized by fungi were considered non-viable. The viability of the remaining seeds was assessed by the tetrazolium chloride test (Moore 1973). Seeds showing positive tetrazolium staining were considered to be viable but dormant. The germination trial was repeated as described above on another set of seeds collected from the same transplant date treatments.
Radicle elongation trial
Seeds of A. theophrasti from each transplant date treatment were removed on the d of germination and transferred to 25 cm x 37.5 cm germination paper (Paper no. 38, Anchor Paper Inc., St. Paul, MN) moistened with distilled water. Seedlings were placed lengthwise along the germination paper with all radicles arranged in the same direction. A second sheet of moistened germination paper was placed directly over the first sheet, leaving the seedlings between the two sheets. The two sheets of germination paper were gently rolled and placed into a 1000-mL glass beaker with the radicles pointing downward. The beakers were then placed back into the growth chambers and subjected to the same conditions used for the germination trial. To keep the germination paper moist, the beaker was filled to a depth of 2.5 cm with distilled water thus allowing for upward capillary movement of water along the germination paper. Each germination sheet contained only seeds which germinated on the same day. Germination sheets for all transplant date treatments were placed in the same beaker and these were replicated three times. Thus, each set of three beakers included all seedlings germinating on the same d. Radicle length (mm) for each seedling was measured daily over a 6-d period from the time of initial transfer.
Statistical analyses
Data for seed weight as well as the germination and radicle elongation experiments were analyzed separately by analysis of variance (ANOVA) using PROC GLM in SAS (SAS 1999). All percentage data from the germination experiment were arcsine square root-transformed to homogenize variances. Data from the radicle elongation experiment did not require transformation. Differences between treatments were established at the P = 0.05 level of significance by using the LSMEANS function of SAS. Regression analysis for reproductive output versus biomass was conducted using PROC REG in SAS (SAS 1999).
Results and discussion
Photoperiod (daylength) during the seed maturation phase of A. theophrasti in Aurora, NY differed between the three transplanting dates (Fig. 1). Seedlings transplanted on 15 May, 4 June, and 30 June entered the seed maturation phase under daylengths of approximately 15, 14, and 13 h, respectively. Photoperiod has been shown to affect the seed coat thickness of seeds with physical dormancy. For example, Evenari et al. (1966) and Gutterman and Heydecker (1973) reported a higher proportion of dormant seeds possessing thicker more impermeable seed coats when plants of the hardseeded desert species Ononis sicula were grown under long (16 h) versus short (8 h) photoperiods. Alternatively, Nurse and DiTommaso (2005) found no influence of natural photoperiod on A. theophrasti seed coat weight or dormancy, but rather attributed differences in both characteristics to competition with maize. In our study, seeds produced under a shorter (peak 13 h) photoperiod had greater dormancy than seeds produced under a longer photoperiod (peak 15 h) (Fig. 2). It remains unclear how other environmental factors such as water, nutrient availability, and daily light intensity may have affected seedcoat thickness or embryo size during seed development.
Figure 1
Photoperiod by month in Aurora, NY for the three Abutilon theophrasti transplant dates 15 May, 4 June, and 30 June.
Seeds produced under shorter photoperiods (13 h) weighed, on average, 1.5 mg less than seeds produced under longer photoperiods (15 and 14 h) (P < 0.0001). For example, the mean weight of seeds (11.3 ± 0.1 and 11.2 ± 0.05 mg) maturing under 15- and 14-h daylengths, respectively did not differ, but mean weight of seeds maturing under the shorter daylengths (< 13 h) was significantly lower (9.5 ± 0.05 mg). Similarly, A. theophrasti planting date also affected the germinability of seeds produced (P < 0.0001) (Fig. 2). The highest levels of germination occurred for seeds from the 4 June transplant date where 98% of seeds germinated by the end of the 14-d trial. The remaining 2% of seeds were viable but dormant. Seeds from the 15 May transplanting and maturing under a longer daylength (15 h) had significantly lower germination (95%). Of the seeds from this treatment not germinating, 3% were viable but dormant. Seeds produced by the 30 June transplants that experienced shorter photoperiods (13 h) had significantly lower seed germination levels (76%) than seeds from the 15 May and 4 June transplants. Of seeds not germinating under the shorter photoperiod treatment, 20% were viable but dormant and 4% of seeds were non-viable. The higher proportion of non-viable seeds obtained for the 30 June transplant treatment may be due to A. theophrasti individuals in this treatment not reaching physiological maturity.
Figure 2
Cumulative germination (%) (± SE) over 14 days for seeds produced in monocultures and planted on three dates in 2000 in Aurora, NY.
Physical dormancy in A. theophrasti regulates the permeability of the seed coat to water (Horowitz and Taylorson 1984; LaCroix and Staniforth 1964; Mulliken and Kust 1970; Warwick and Black 1988). Seed coat permeability has previously been linked to preci-pitation levels experienced by the maternal plant during the maturation and seed development phases of growth (Fenner 1991). The final 2 mo of the growing season experienced below average rainfall and thus, A. theophrasti individuals transplanted on 30 June (i.e. 13 h peak photoperiod) experienced drier condi-tions during their seedling growth phase than transplants from the 15 May and 4 June plantings. This difference in water stress may explain the greater dormancy of seeds produced by the 30 June transplants (Nurse and DiTommaso 2005). Average temperatures during the final 2 mo of the growing season were also higher than the 30-yr average (Table 1). This may have contributed to smaller seed size and less vigourous seedling growth. Above-ground biomass of 30 June transplants was lower than for the other two transplant dates (Fig. 3). Total seed production was positively correlated to final above-ground biomass regardless of transplant date.
Table 1
Mean precipitation (and 45-yr average) and temperatures (and 30-yr average) for months ranging from Abutilon theophrasti seedling transplanting to seed harvest in Aurora, NY in 2000
Figure 3
Regression of above-ground biomass versus total seed production in Abutilon theophrasti monoculture at three transplanting dates in 2000.
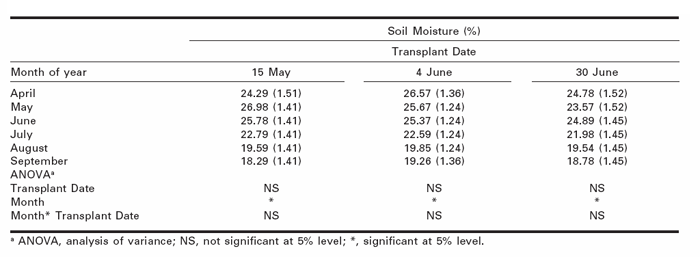
Soil moisture content (volumetric) declined throughout the growing season (Table 2) and moisture levels in July through September were significantly lower than between April and June (P < 0.05). None-theless, soil moisture in the second half of the grow-ing season was not below the permanent wilting point for this soil type (Nurse and DiTommaso 2005). Although it remains unclear how small reductions in soil water content during seed maturation may influence the dormancy status of seeds produced. It is known that the pore near the chalazal opening is sensitive to seed moisture content and is responsible for some degree of dormancy regulation (LaCroix and Staniforth 1964).
Table 2
Percentage soil moisture as measured by time domain reflectrometry (TDR) for each transplant date over the entire growing season.
In a 2-yr field study in Ames, Iowa, USA, examining the effect of shade on A. theophrasti growth, seed output and seed dormancy, Bello et al. (1995) con-cluded that precipitation differences between the 2 yr did not influence the proportion of seeds that were dormant. However, in the 2 yr of their study, total precipitation for the mo of May and June which cor-responds to the peak vegetative growth phase of A. theophrasti (i.e. 446.3 and 98.5 mm; 30-yr average = 94.8 mm) were greater than the 60 mm received at our site during peak vegetative growth of the 30 June transplants. Thus, plants in the Bello et al. (1995) study were likely not subjected to as severe water-stressed conditions during vegetative development as plants in this study.
The lengths of seedling radicles after 6 d of growth were highly variable within the latter two transplant date treatments (Fig. 4). Seedlings originating from seeds collected from 4 June transplants showed the greatest variability in length with some seedlings having radicles in excess of 200 mm but others had lengths below 10 mm. For this reason, the length of radicles did not differ significantly (P > 0.05) among seedlings within the three transplant dates (Fig. 4).
Figure 4
Radicle elongation (± SE over 6 days for Abutilon theophrasti seeds collected from monocultures and germi-nating on days 1, 2, 3, and 4 of the germination trial.
Early germinating seeds of A. theophrasti (i.e. within the first 6 d) produced under the different photoperiods did not give rise to seedlings with different initial vigour despite differences in mean seed weight among the treatments. It had been expected that seedlings produced from seeds that had matured under more favourable growth conditions (i.e. longer photo-periods) would have greater initial growth since maternal plants would have allocated more resources to seed structures such as the embryo and endosperm. The trend of decreasing radicle length as photoperiod declined is consistent with this view, but the high variability in results even within the same treatment suggests that this relationship was weak at best. Interestingly, Weinig (2000) also reported that seedling elongation in A. theophrasti was little affected by the growing environment of maternal plants, although seedling vigour was assessed at a later growth stage than our study. However, the fact that we monitored radicle growth for only 6 d after germination may have also influenced results. Finally, anecdotal observations from the 14-d germination experiment indicate that seeds germinating early (i.e. from 1-3 d) might be more vigourous than seeds germinating late (i.e. from 4-6 d).
In conclusion, seed dormancy increased in A. theophrasti individuals maturing under shorter (peak 13 h) versus longer (peak 15 h) photoperiods. These findings did not support our original hypothesis and may be due to the drought stress experienced by the 30 June transplants and the re-allocation of maternal resources to the seed coat possibly increasing its thickness and impermeability to water. Nurse and DiTommaso (2005) have shown that seed dormancy is not affected by photoperiod in velvetleaf in competition with maize. More in-depth research is needed to better assess the effect of photoperiod on seed coat thickness in A. theophrasti and other species. Seedling vigour did not differ with planting date despite the significantly lighter seeds produced by plants subjected to the shorter photoperiod. It is clear that the daylength experienced by A. theophrasti plants grown in monoculture during vegetative growth and seed development has a significant effect on the dormancy status of seeds produced. Although A. theophrasti most frequently infests crop production systems, examining the effects of planting date in monocultures as done in this study is applicable to field situations where plants often grow in pure stand within fallow areas bordering crop fields or in areas of the field with poor crop establishment. Future studies that are conducted over a broader range of environments and include testing of A. theophrasti seeds collected from more than one population may improve our understanding of how drought and other environmental variables impact seed germinability in this species.
Parties annexes
Acknowledgements
The authors thank Drew Zwart and Karen Joslin for technical assistance. We also thank Daniel C. Brainard, Robert S. Gallagher, Ralph L. Obendorf, Ray B. Taylorson and two anonymous reviewers for reviewing an earlier draft of this manuscript. Funding for this work was provided by Hatch funds – 125402 to A. DiTommaso from the NY State Agric. Exp. Stn.
References
- Baskin, C.C, and J.M. Baskin. 1998. Seeds: Ecology, biogeography, and evolution of dormancy and germi-nation. Academic Press, San Diego. CA. 666 pp.
- Baskin, J.M., and C.C. Baskin. 2004. A classification system of seed dormancy. Seed Sci. Res. 14 : 1-6.
- Bello, I.A., M.D.K. Owen, and H.M. Hatterman-Valenti. 1995. Effect of shade on velvetleaf (Abutilon theophrasti) growth, seed production, and dormancy. Weed Technol. 9 : 452-455.
- Evenari, M., D. Koller, and Y. Gutterman. 1966. Effects of the environment of the mother plant on germination by control of seed coat impermeability to water in Ononis sicula Guss. Aust. J. Biol. Sci. 19 : 1007-1016.
- Fenner, M. 1991. The effects of the parent environment on seed germinability. Seed Sci. Res. 1 : 75-84.
- Gutterman, Y.2000. Maternal effects on seeds during development. Pages 59-84 in M. Fenner (ed.), Seeds: The ecology of regeneration in plant communities. 2nd ed. CABI Publishing, Wallingford, U.K.
- Gutterman, Y., and W. Heydecker. 1973. Studies of the surfaces of desert plant seeds. I. Effect of day length upon maturation of the seedcoat on Ononis sicula Guss. Ann. Bot. 37 : 1049-1050.
- Horowitz, M., and R.B. Taylorson. 1984. Hardseededness and germinability of velvetleaf (Abutilon theophrasti) as affected by temperature and moisture. Weed Sci. 32 : 111-115.
- Horowitz, M., and R.B. Taylorson. 1985. Behaviour of hard and permeable seeds of Abutilon theophrasti Medic. (velvetleaf). Weed Res. 25 : 363-372.
- Huang J.Z., A. Shrestha, M. Tollenaar, W. Deen, H. Rahimian, and C.J. Swanton. 2000. Effects of photoperiod on the phenological development of redroot pigweed (Amaranthus retroflexus L.). Can. J. Plant Sci. 80 : 929-938.
- LaCroix, L.J., and D.W. Staniforth. 1964. Seed dormancy in velvetleaf. Weeds 12 : 171-174.
- Moore, R.P. 1973. Tetrazolium staining for assessing seed quality. Pages 347-366 in W. Heydecker (ed.), Seed ecology. Butterworths, London.
- Mulliken, J.A., and C.A. Kust. 1970. Germination of velvetleaf. Weed Sci. 18 : 561-564.
- Nurse, R.E., and A. DiTommaso. 2005. Corn competition alters the germinability of velvetleaf (Abutilon theophrasti) seeds. Weed Sci. (in press).
- Oliver, L.R. 1979. Influence of soybean (Glycine max) planting date on velvetleaf (Abutilon theophrasti) competition. Weed Sci. 27 : 183-188.
- Patterson, D.T. 1995. Effects of photoperiod on reproductive development in velvetleaf (Abutilon theophrasti). Weed Sci. 43 : 627-633.
- Statistical Analysis Systems Institute. 1999. SAS/STAT User’s Guide, Version 7-1. Cary, NC. 1030 pp.
- Stirling, K.J., R.J. Clark, P.H. Brown, and S.J. Wilson. 2002. Effect of photoperiod on flower bud initiation and development in myoga (Zingiber mioga Roscoe). Sci. Hortic. 95 : 261-268.
- Warwick, S.I., and L.D. Black. 1988. The biology of Canadian weeds. 90. Abutilon theophrasti. Can. J. Plant Sci. 68 : 1069-1085.
- Weinig, C. 2000. Limits to adaptive plasticity: temperature and photoperiod influence shade-avoidance responses. Am. J. Bot. 87 : 1660-1668.
Liste des figures
Figure 1
Photoperiod by month in Aurora, NY for the three Abutilon theophrasti transplant dates 15 May, 4 June, and 30 June.
Figure 2
Cumulative germination (%) (± SE) over 14 days for seeds produced in monocultures and planted on three dates in 2000 in Aurora, NY.
Figure 3
Regression of above-ground biomass versus total seed production in Abutilon theophrasti monoculture at three transplanting dates in 2000.
Figure 4
Radicle elongation (± SE over 6 days for Abutilon theophrasti seeds collected from monocultures and germi-nating on days 1, 2, 3, and 4 of the germination trial.
Liste des tableaux
Table 1
Mean precipitation (and 45-yr average) and temperatures (and 30-yr average) for months ranging from Abutilon theophrasti seedling transplanting to seed harvest in Aurora, NY in 2000
Table 2
Percentage soil moisture as measured by time domain reflectrometry (TDR) for each transplant date over the entire growing season.