Résumés
Abstract
Biosorbents, especially those derived from seaweed (macroscopic algae) and alginate derivatives, exhibit high affinity for many metal ions. Because biosorbents are widely abundant (usually biodegradable) and less expensive than industrial synthetic adsorbents, they hold great potential for the removal of toxic metals from industrial effluents. Various studies have demonstrated the efficiency of living and non-living micro-organisms, such as bacteria, yeasts, moulds, micro-algae, cyanobacteria and biomass from water treatment sewage to remove metals from solution. Several types of organic and inorganic biomass have also been used as sorbent materials. In addition, by-products from the forestry industry, as well as agriculture waste and natural sorbents, have also been studied. This paper reviews and summarizes some key recent developments in these areas and it describes and discusses some specific applications of selected natural sorbents.
Key Words:
- Metal,
- removal,
- adsorption,
- alginate,
- seaweed,
- natural sorbent,
- wastewater,
- effluent,
- treatment
Résumé
Les biosorbants, particulièrement ceux préparés à partir des algues macroscopiques et des dérivés d’alginate, démontrent une très bonne capacité d’adsorption des ions métalliques toxiques. Ces biosorbants étant facilement disponibles (biodégradable) et moins coûteux que les adsorbants (industriels) synthétiques, ils présentent un grand potentiel d’utilisation pour l’enlèvement des métaux toxiques des effluents industriels. Les récents développements dans ce domaine ont été revus et font l’objet de la présente synthèse. Des applications spécifiques sont décrites et discutées.
Diverses technologies sont disponibles pour enlever les métaux des effluents industriels tels que la précipitation (sous forme d’hydroxydes ou de sulfures), la coprécipitation, l’adsorption, l’extraction par solvant, la cémentation, l’électrodéposition, l’électrocoagulation, l’échange d’ions et les technologies de séparation membranaire. Néanmoins, la plupart de ces techniques présentent des coûts d’exploitation élevés et, dans certains cas, sont limitées en terme de rendement d’élimination des métaux. Dans ce contexte, l’utilisation d’adsorbants naturels (dérivés de matière organique ou inorganique) constitue une alternative intéressante aux produits synthétiques. De nombreux articles ont d’ailleurs été publiés au cours des dernières années faisant état de la performance d’une grande variété d’adsorbants naturels pour enlever les métaux des effluents.
Plusieurs espèces d’algues marines ont aussi démontré des propriétés pour adsorber les métaux, mais les algues marines brunes, telles que Sargassum et Ascophyllum semblent avoir la plus grande capacité de rétention des métaux, à cause de leur grande concentration en polysaccharides. L’intégrité physique des algues est également importante, ceci afin de prévenir leur désintégration pendant les processus d’adsorption. Afin d’améliorer la stabilité et les propriétés mécaniques des algues fraîches, diverses méthodes ont été suggérées : i) greffage dans des polymères synthétiques; ii) incorporation dans des matériaux inorganiques; iii) liaison sur un support adéquat; et iv) séquestration par un agent de liaison.
L’acide alginique est un polymère naturel se trouvant dans les algues brunes. Ce polymère est extrait en traitant les algues avec une solution de carbonate de sodium, puis l’acide alginique est précipité, ou converti en sel d’alginate de calcium. Lorsque l’acide alginique réagit avec des ions polyvalents, tel que le calcium, une séquestration se produit procurant un gel d’alginate ayant des forces structurales significatives. L’alginate de calcium peut être préparé sous plusieurs formes, telles que des billes, de la poudre, des membranes, des fibres ou des supports d’immobilisation cellulaire. Les billes sont particulièrement intéressantes du point de vue de leur application et de leur réutilisation.
L’utilisation des algues marines en tant que procédé d’enlèvement des métaux doit tenir compte de plusieurs considérations techniques. Les systèmes de biosorption utilisent les biomasses sous forme solide en un procédé conventionnel de contact solide-liquide et, dans certains cas, les systèmes comprennent plusieurs étapes de biosorption et de désorption. L’effluent à traiter peut entrer en contact avec la biomasse selon un procédé en mode discontinu, semi-continu ou continu. Une fois saturés en métaux lourds, les adsorbants peuvent être disposés de façon sécuritaire, ou être réutilisés après élution des métaux. Dans ce cas, la plupart des métaux lourds (Cd, Co, Cu, Mn, Pb, Zn) peuvent être élués à l’aide d’acides dilués (chlorhydrique, sulfurique, nitrique) ou de solutions salines concentrées. Certains métaux qui sont moins dépendants du pH d’adsorption (Au, Ag, Hg) ne peuvent être élués en utilisant un acide dilué. Des solutions de thiourée ou de mercaptol peuvent être utilisées pour l’or et l’acétate de sodium pour la récupération de l’argent. La combustion des algues est également possible, néanmoins, elle n’est envisageable que si l’adsorbant est peu dispendieux et grandement disponible.
Plusieurs types de biomasses organiques ou inorganiques peuvent être utilisés comme matériaux adsorbants. Des études ont démontré l’efficacité des microorganismes vivants ou morts incluant les bactéries, les levures, les moisissures, les microalgues, les cyanobactéries et les biomasses issues du traitement des eaux usées (boues d’épuration). Les rejets de l’industrie forestière, incluant les sciures et les écorces de bois riches en lignine et en tannins, ont été également étudiés de façon intensive. Certaines plantes aquatiques (Ceratophyllum demersum, Lemna minor, Myriophyllum spicatum) ont également été évaluées pour leur capacité en phytofiltration et phytoassainissement. D’autres études ont été effectuées sur la performance de fixation des métaux de la chitine, cette dernière étant un biopolymère naturel très abondant, lequel est classé second après la cellulose en terme d’abondance. Ce biopolymère se retrouve largement dans l’exosquelette des crustacés et des coquillages. Le chitosan est produit en effectuant la dé-acétylation de la chitine en milieu alcalin. La mousse de tourbe, les déchets d’agriculture (résidus de thé et de café, pelures de certains légumes, écailles de noix, d’arachides, de cacao) et divers autres adsorbants de nature inorganique (sable, argile, oxyde, zéolites) ont également été étudiés pour la récupération des métaux en solution.
D’un point de vue économique, plusieurs méthodes existent pour traiter les eaux usées. La sélection d’une méthode dépend de plusieurs critères, tels que la compatibilité avec les opérations existantes, les coûts d’exploitation, la flexibilité des procédés afin de pouvoir traiter des variations de charges hydrauliques et de concentrations de contaminants. La méthode doit être aussi fiable, robuste et simple d’utilisation. Dans certains cas, des économies substantielles peuvent être réalisées en faisant appel à l’adsorption des métaux sur des biomasses, comparativement aux procédés conventionnels, tel que la précipitation des métaux.
Mots clés:
- métal,
- enlèvement,
- adsorption,
- alginate,
- algue,
- eau usée,
- sorbant naturel,
- effluent,
- traitement
Corps de l’article
Introduction
The preservation of the environment has become increasingly important in view of the ecological problems brought about by industrialization and urbanization. Lakes and rivers are particularly vulnerable to contamination as a result of the discharge of large quantities of effluents from industries and municipalities. The presence of heavy metals, such as cadmium, chromium, cobalt, copper, lead, mercury, nickel, silver, tin and zinc in rivers and watercourses may cause serious health problems to living organisms (ALLOWAY and AYERS, 1993; WASE and FORSTER, 1997).
Consequently, the norms and regulations imposed on industrial effluents are becoming increasingly stringent. These restrictions stem largely from recent advances in the understanding of the behaviour of heavy metals in the environment. Being non-biodegradable, toxic metals tend to accumulate in lower plants and animals, thereby entering the food chain (CHANG et al., 2003; LIPPMANN, 2000; WATTS, 1998).
Various technologies are available to remove metal ions from industrial effluents, including precipitation (usually as metal hydroxide or sulphide) and coprecipitation, sorption, solvent extraction, cementation, electrodeposition, electrocoagulation, ion exchange, and membrane technology (BLAIS et al., 1999; BROOKS, 1991; CHMIELEWSKI et al., 1997; PATTERSON, 1989). However, most of these techniques require expensive, usually toxic, reagents, and this fact significantly increases the capital and operating costs. In this context, biosorbents (i.e., ion exchangers and adsorbents derived from organic matter) present an attractive alternative to synthetic and chemical products because they are widely available, generally biodegradable and relatively inexpensive.
The main characteristics and past applications of biosorbents have been summarized and discussed by several researchers (ATKINSON et al., 1998; BABEL and KURNIAWAN, 2003; BAILEY et al., 1999; FISET et al., 2000; KUYUCAK and VOLESKY, 1988; VEGLIO and BEOLCHINI, 1997; VOLESKY, 1990; VOLESKY and HOLAN, 1995; WASE and FORSTER, 1997). This present review summarizes the recent research carried out on the use of biosorbents, especially those derived from seaweeds, as substrates to remove heavy metal from solutions and effluents. The various systems, mechanisms and results presented in the scientific literature are analyzed and compared.
1. Seaweed Sorbents
1.1 Seaweed-derived sorbents
The ability of biosorbents derived from marine algae to adsorb metal ions has been demonstrated by several researchers (LEE and VOLESKY, 1997; LEUSCH et al., 1995; VEGLIO and BEOLCHINI, 1997; VOLESKY and HOLAN, 1995; VOLESKY and PRASETYO, 1994; WILSON and EDYVEAN, 1994).
According to FAO (2002), the world aquaculture production of brown, red and green seaweeds in 2002 was approximately 5, 2.5 and 0.018 Megatons (wet basis), respectively. Whereas all these seaweed species exhibit good metal adsorption properties, the brown marine algae (Sargassum and Ascophyllum) have the highest capacity for heavy metal ions because of their high polysaccharide content (VOLESKY and HOLAN, 1995). Tables 1 and 2 summarize the research carried out on the adsorption of metal ions using various seaweed species.
Table 1
Studies on the metal sorption using seaweeds
Études portant sur l’adsorption des métaux par utilisation d’algues macroscopiques
References |
Seaweeds |
Metals |
Studied parameters |
|---|---|---|---|
Aravindhanet al. (2004) |
Turbinaria sp. |
Cr(III) |
Pre-treated with H2SO4, CaCl2 and MgCl2 |
Axtellet al. (2003) |
Microspora sp. |
Ni(II), Pb(II) |
Batch and semi batch process, kinetic study |
borba et al. (2006) |
Sargassum filipendula |
Ni(II) |
Pre-treatment, fixed bed column, mathematical modeling |
cepriáet al. (2006) |
Ulva rigida |
Au(III), Hg(II), Ag(I), |
Biomass modified electrode, factors affecting biosorption |
Chaisuksant (2003) |
Gracilaria fisheri (red marine algae) |
Cd(II), Cu(II) |
Langmuir model, effect of pH, pre-treated with CaCl2. |
Cossichet al. (2004) |
Sargassum sp. |
Cr(III) |
Fixed-bed column, mass balance |
Da Costa and De França (1996) |
Codium sp., Colpomenia sp., Gelidium sp., Padina sp., Sargassum sp., Ulva sp. |
Cd(II) |
Langmuir and Freundlich models, ion-exchange mechanism, effect of pH |
Da Costaet al. (1996) |
Sargassum sp. |
Al(III), Ca(II), Cd(II), Mg(II), Na(I), Zn(II) |
Synthetic and natural effluents, kinetic |
Gonget al. (2005) |
Spirulina maxima |
Pb(II) |
pH, contact time, biomass concentration, Freundlich model, desorption, pre-treated with CaCl2. |
Guptaet al. (2001) |
Spirogyra sp. |
Cr(VI) |
Batch sorption, kinetic studies, effect of pH, Langmuir model |
Holanet al. (1993) |
Ascophyllum nosodum, Fucus vesiculosus |
Cd(II) |
Cross-linking, desorption |
Holan and Volesky (1994) |
Ascophyllum nosodum, Fucus vesiculosus |
Ni(II), Pb(II) |
Langmuir model, effect of pH, crosslinked with formaldehyde, bis(ethenyl)sulfone and 1-chloro-2,3-epoxypropane |
Kaewsarn and YU (2001) |
Padina sp. |
Cd(II) |
Batch and column experiments, kinetic studies, effect of pH |
Kaewsarnet al. (2001) |
Durvillaea potatorum |
Cu(II) |
Co-ions effect (light and heavy metals, EDTA) |
Kaewsarn (2002) |
Padina sp. |
Cu(II) |
Langmuir isotherm, kinetic studies, effect of pH, batch and fixed-bed experiments |
khani et al. (2006) |
Cystoseira indica |
U(VI) |
Batch sorption, kinetic modelling, effect of pH, protonated algae, Ca-pretreated algae |
Kratochvillet al. (1998) |
Sargassum sp. |
Cr(III), Cr(VI) |
Protonated seaweed, pH optimization |
Kuyucak and Volesky (1988) |
Ascophyllum nodosum, Chondrus crispus, Halimeda opuntia, Palmaria palmata, Porphyra tenera, Sargassum natans |
Ag(I), Au(III), Cd(II), Co(II), Cu(II), Pb(II), U(VI), Zn(II) |
Algal biomass, dead and living yeast, comparison with activated carbon, anion exchange resin (IRA-400) and cations exchange resin (Duolite-C20) |
kumaret al. (2006) |
Ulva fasciata sp. |
Cu(II) |
Effect of pH and algae concentration, effect of particle size, adsorption kinetics, pseuso-first and pseudo-second models, adsorption equilibrium |
Lauet al. (2003) |
Ulva lactuca |
Cu(II), Ni(II), Zn(II) |
Langmuir isotherm, effect of cations and anions, kinetic study, effect of pH, seaweed biomass concentration, effect of reaction time,desorption |
Lee and Volesky (1997) |
Sargassum fluitans |
Al(III), Ca(II), K(I), Mg(II), Na(I) |
Light metals affinity, proton uptake |
Leuschet al. (1995) |
Ascophyllum nodosum Sargassum fluitans |
Cd(II), Cu(II), Ni(II), Pb(II), Zn(II) |
Crosslinked with formaldehyde, glutaraldehyde and embedded in polyethylene imine, Langmuir, Freundlich and Dubinin-Radushkevich models, effect of particle size |
lodeiroet al. (2006) |
Cystoseira baccata |
Cd(II), Pb(II) |
Kinetic experiments, temperature effect, Langmuir-Freundlich model, effect of salinity on metal uptake, FTIR analysis |
luoet al. (2006) |
Laminaria japonica |
Pb(II) |
Chemical modification, Langmuir isotherm, effect of pH, effect of solid/liquid ratio. |
Matheickal et al. (1997) |
Ecklonia radiata |
Cu(II) |
pH effect, effect of EDTA, acetate, nitrate and chloride on metal uptake, packed bed system |
Matheickal and YU (1997) |
Phellinus badius |
Pb(II) |
Langmuir isotherm, kinetic studies, effect of pH, batch and fixed-bed experiments |
naja and Volesky (2006) |
Sargassum fluitans |
Cu(II), Zn(II), Cd(II) |
Equilibrium and sorption models |
Oferet al. (2003) |
Padina pavonia, Sargassum vulgaris |
Cd(II), Ni(II) |
Kinetic studies, desorption studies, Langmuir isotherm |
Parket al. (2004) |
Ecklonia sp. |
Cr(III), Cr(VI) |
Redox reaction with the biomass, thermal treated biomass, SEM, BET, FTIR characterization |
Prasheret al. (2004) |
Palmaria palmate (red algae) |
Cd(II), Cu(II), Ni(II), Pb(II), Zn(II) |
Freundlich, Langmuir and Brunauer Emmer and Teller (BET) models, effect of contact time, pH, initial concentration and temperature |
Senthilkumaret al. (2006) |
Gracilaria crassa, Gracilaria edulis, Hypnea valentiae, Ulva lactuca, Ulva reticula, Codium tomentosum, Chaetomorpha antennina, Turbinaria conoides, turbinaria ornata, Sargassum polycystium |
Zn(II) |
Batch experiments, Langmuir, Freundlich, Redlich-Peterson and Sips models, columns experiments, biosorption kinetics, sorption thermodynamics, influence of co-ions, desorption studies |
tsui et al. (2006) |
Sargassum hemiphyllum |
Ag(I), As(V), Cd(II), Co(II), Cd(II), Cr(III), Cr(VI), Mn(II), Ni(II), Pb(II), Zn(II) |
Ca-treated biomass, different ionic strengths, binding mechanism |
Volesky (1994) |
Sargassum natans, Sargassum fluitans, Sargassum vulgaris, Ascophyllum nodosum, Palmaria palmata, Chondrus crispus, Halimeda opuntia, Fucus vesiculosis, Padina gymnospora, Codium taylori |
Cd(II), Pb(II) |
|
Yuet al. (1999) |
Ascophyllum nodosum, Lessonia flavicans, Lessonia nigresenseLaminaria japonica, Laminaria hyperbola, Ecklonia maxima, Ecklonia radiate, Durvillaea potatorum |
Cd(II), Cu(II), Pb(II), U(VI) |
Langmuir isotherm, radionuclides, effect of grown media |
Table 2
Recent studies on the adsorption capacities (mg/g) of marine algae for selected heavy metals.
Études récentes sur la capacité d’adsorption (mg/g) des l’algues marines pour des métaux lourds sélectionnés.
References |
Sorbents |
Cd(II) |
Cu(II) |
Cr(III) |
Ni(II) |
Zn(II) |
|---|---|---|---|---|---|---|
ARAVINDHAN et al. (2004) |
T. ornata |
|
|
31 |
|
|
CHAISUKANT (2003) |
G. fisheri |
71 |
46 |
|
|
|
COSSICH et al. (2004) |
Sargassum sp. |
|
|
68 |
|
|
KUMAR et al. (2006) |
Ulva fasciata sp. |
|
26.9 |
|
|
|
LAU et al.(2003) |
Ulvas sp. 1 Ulva lactuca Ulva sp. 3 |
|
47 55 51 |
|
10 19 16 |
34 44 31 |
LODEIRO et al. (2004) |
S. muticum |
154 |
|
|
|
|
LODEIRO et al. (2006) |
C. baccata |
101 |
|
|
|
|
OFER et al. (2003) |
S. vulgaris P. pavonia |
135 88 |
|
|
62 34 |
|
PAVASANT et al. (2006) |
Caulerpa lentillifera |
1.59 |
5.56 |
|
|
2.66 |
KLIMMEK et al. (2001) compared the efficiency of thirty strains of algae for their abilities to extract cadmium, lead, nickel and zinc from aqueous solution. These researchers found that the cyanophyceae Lyngbya taylorii exhibited high uptake capacities for the four metals. Similarly, VOLESKY and HOLAN (1995) provide some 23 examples of algal biomass metal adsorption.
The physical integrity of algae is important to prevent them from disintegrating during the sorption process. HOLAN et al. (1993) summarized various techniques used to improve the stability and the mechanical properties of fresh biopolymers:
Grafting into synthetic polymers;
Entrapment into inorganic material;
Binding to a suitable carrier; and
Cross-linking.
1.2 Algins and alginates derivatives
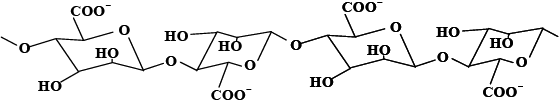
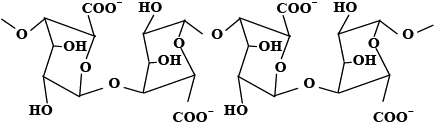
Algins are salts of alginic acid, a natural polymer found in brown algae (Phaeophyceae). This polymer is extracted by treating the seaweed with a sodium carbonate solution and recovered by precipitation as alginic acid and afterward as the sodium salt. The alginic acid molecules have a complicated structure. Figure 1 shows two of the main segments found in alginic acid. The abundance of carboxylic, hydroxyl and oxo groups gives alginic acid and alginate salts strong chelating properties for metal ions.
Figure 1
Main segments of alginic acid: A) a poly(D-mannuronosyl segment and B) a poly(L-guluronosyl) segment.
Principales parties de l’acide alginique : A) une portion poly(D-mannuronosyl, et B) une portion poly(L‑guluronosyl).
A
B
When alginic acid reacts with polyvalent ions, such as calcium, a cross-linking effect takes place, which gives the resulting alginate gel a significant structural strength (NESTLE and KIMMICH, 1996). The cross-linking is caused by a polyvalent ion binding two or more carboxylic groups on adjacent polymer chains, and this can be accompanied by chelation of the ion by the hydroxyl and carboxyl groups of the polymer chains (SHIMIZU and TAKADA, 1997).
The alginate products are not only used for metal removal, but also for other commercial applications, including some in the food industry (HOLAN et al., 1993; RENN, 1997). The main advantage of using algae or biomass derivatives is that they do not require nutrients and they are resistant to the physical-chemical properties of heavy metal solutions (ARAÚJO and TEIXEIRA, 1997). Alginate products have been used as supporting substrate for a variety of active agents, including microorganisms, algae (AL-RUB et al., 2004; SINGHAL et al., 2004), chitosan (GOTOH et al., 2004; HUANG et al., 1996), activated sludge (WANG et al., 2004), cellulose and humic acid (MISRA and PANDEY, 2001). Tables 3 and 4 present alginate derivatives studied for their capacity to adsorb different metals.
Table 3
Studies on the metal sorption using alginate products.
Études portant sur l’adsorption des métaux sur les produits d’alginate.
References |
Sorbents |
Metals |
Studied parameters |
|---|---|---|---|
Al-Rubet al. (2004) |
Alginate beads |
Ni(II) |
Effect of immobilized algae cells (living and death), effect of pH and initial concentration, alginate beads, Langmuir model, sorption desorption cycle |
Aksuet al. (1998) |
Ca-alginate beads agarose, Chlorella vulgaris |
Cu(II) |
Packed-bed column, flow rates |
Chenet al. (1993) |
Alginate beads |
Cu(II) |
Linear absorption model, diffusion coefficient, density of alginate |
Cristet al. (1994) |
Vaucheria, Rhizoclonium, Ca-alginate powder |
Ag(II), Al(III), Ba(II), Cd(II), Cu(II), La(III), Mg(II), Pb(II), Sr(II) Zn(II) |
Ion exchange constant, rate of removal of sorbed metal, ion exchange model |
Fourest and Volesky (1997) |
Alginate extraction from Sargassum fluitans, Ascophyllum nodosum, Fucus vesiculosus, Laminaria japonica |
Ca(II), Cd(II), Cu(II), Pb(II), Zn(II) |
Characterization of physical properties of alginate by potentiometric titration and 13C NMR, metal binding |
Gotohet al. (2004) |
Alginate gel beads |
Cu(II), Mn(II) |
Doped alginate beads with cyanogens bromide and 1,6-diaminohexane, FTIR and SEM characterization |
Hirai and Odani (1994) |
Alginic acid film, sodium alginate film, alginate-Co complex |
Co(II) |
Absorption, desorption, diffusion coefficient, film characterization |
Huanget al. (1996) |
Alginate and chitosan beads |
Cu(II), Ni(II) |
Metal selectivity, particle size, kinetic, isotherm, effect of pH |
IBÁÑEZ and Umetsu (2002) |
Protonated alginate beads |
Co(II), Cr(III), Cu(II), Ni(II), Zn(II) |
Metal uptake, beads morphology, effect of protonation, effect of: ionic strength, pH and protonation |
IBÁÑEZ and Umetsu (2004) |
Protonated dry alginate beads |
Cr(III) |
Batch tests, effect of pH, mechanism, EPMA-EDX analysis |
Janget al. (1995) |
Alginate beads |
Co(II), Cu(II) |
In-situ crosslinking, metal selectivity, fluidized bed reactor, Langmuir model |
Janget al. (1999) |
Alginate beads |
Cu(II), Zn(II) |
In-situ crosslinking, extended Langmuir model, binding group density, binding stability constant |
Jeonet al. (2002) |
Carboxylated alginic acid |
Pb(II) |
Carboxylated alginic acid using KMnO4, FTIR and 13C NMR characterization, elemental analysis, desorption |
Jeonet al. (2005) |
Carboxylated alginic acid |
Cu(II), Pb(II) |
Effect of ionic strength and organic material effect, desorption |
Karagunduzet al. (2006) |
Dried alginate beads |
Cu(II) |
Kinetic sorption, equilibrium experiment, surfactant entrapped dried alginate beads. |
Lu and Wilkins (1996) |
Sacharomyces cerevisiae immobilized in alginate gels |
Cd(II), Cu(II), Zn(II) |
Caustic treatment, metal desorption, biosorbent reactivation |
Nestle and Kimmich (1996) |
Alginate beads |
Cu(II) |
NMR analyses, spatial distribution, diffusion coefficient |
PAPAGEORGIOUet al. (2006) |
Alginate beads, alginate extracted from Laminaria digitata |
Cu(II), Cd(II), Pb(II) |
Alginate beads characterization, batch metal uptake, Langmuir, Freundlich and Sips models, kinetic model, batch kinetic model |
Park and Chae (2004) |
Alginate beads, alginate capsules, alginate gel coated |
Pb(II) |
Regeneration of alginate adsorbent, SEM characterization |
Seki and Suzuki (1996) |
Alginic acid and humic acid membranes |
Pb(II) |
Equilibrium parameters, complexation model |
Shimizu and Takada (1997) |
Alginate fibers |
Bi(III), Cu(II), Pb(II), Sr(II) |
Effect of nitric acid, fibers characterization |
Singhalet al. (2004) |
Chlorella pyrendoidosa impregnated beads Ca-alginate |
U(IV), U(VI) |
Column, desorption, kinetic, FTIR characterization |
Veglioet al. (2002) |
Ca-alginate beads |
Cu(II) |
Effect of pH, Langmuir isotherm |
Table 4
Recent studies on the adsorption capacities (mg/g) of alginate for selected heavy metals.
Études récentes sur la capacité d’adsorption (mg/g) de l’alginate pour des métaux lourds sélectionnés.
References |
Sorbents |
Cu(II) |
Cr(III) |
Cr(VI) |
Ni(II) |
Pb(II) |
|---|---|---|---|---|---|---|
AL-RUB et al. (2004) |
Alginate beads Free dead algal cell Immobilized dead algal cells |
|
|
|
26 14 31 |
|
BAJPAI et al. (2004) |
Bio-polymeric (Ca and gelatin) |
|
|
0.83 |
|
|
IBÁÑEZ and UMETSU (2004) |
Protonated dry alginate beads |
|
112 |
|
|
|
Ngomsiket al. (2006) |
Magnetic alginate microcapsule |
|
|
|
26 |
|
OZDEMIR et al. (2005) |
Alginate beads Alginate-Extracellular polysaccharide |
96 126 |
|
|
59 71 |
|
PARK and CHAE (2004) |
Alginate beads Alginate capsule |
|
|
|
|
450 1540 |
Calcium alginate may be prepared in various forms, such as beads, powder (CRIST et al., 1994), membranes (HIRAI and ODANI, 1994; TOTI and AMINABHAVI, 2002) or fibers (SHIMIZU and TAKADA, 1997; WILLIAMS and EDYVEAN, 1997) and can be used as cell-immobilization support (IBÁÑEZ and UMETSU, 2002). Bead particles have practical advantages in terms of applicability to a wide variety of process configuration and reusability (GOTOH et al., 2004). Also, the alginate beads may be protonated (IBÁÑEZ and UMETSU, 2002, 2004) or doped with another metallic ion to obtain various bead properties (MIN and HERING, 1998). GOTOH et al. (2004) improved the mechanical strength and resistance to chemical and microbial degradation without affecting adsorption capacity by cross-linking the alginate beads with 1,6-Diaminohexane. Producing alginate gels “in-situ” is also feasible when a high concentration of metal ions is present in solution (ARAÚJO and TEIXEIRA, 1997; JANG et al., 1999).
Various chemical treatments may be applied on alginic acid in order to increase metal uptake capacity such as carboxylation, phosphorylation, and sulfonation (JEON et al., 2002), although these treatments tend to increase the cost of the resulting product.
ARAÚJO and TEIXEIRA (1997) studied the transport properties of Cr(III) on alginate beads using the Linear Adsorption model and the Shrinking Core model. For low Cr(III) concentration, ion exchange was the rate-controlling mechanism and the experimental results fit well with the Shrinking Core model. At higher concentrations, however, the Linear Adsorption model was a better fit for the experimental results and ionic exchange was no longer the main mechanism of sorption. The study of CHEN et al. (1993) indicated that Linear Adsorption model was preferable for the Cu calcium alginate gel.
1.3 Algal biosorption systems
Engineering considerations are very important during the development of an algal-based sorption system. All biosorption systems used biomass in solid form in a basic solid-liquid contact process, with, in certain cases, cycling of the process through biosorption and desorption stages (GARNHAM, 1997). The effluent to treat would make contact with the biosorbent in a batch, semi-continuous or continuous flow system. The following reactor types have been described by BANKS (1997) as potential biosorption systems:
Conventional stirred tank reactors;
Packed bed reactors (upflow and downflow);
Expanded bed reactors;
Fluidised bed reactors;
Airlift reactors.
In the cases of algal-based processes using actively growing biomass these can also be based on ponds, lagoons, streams and artificial stream meander units (VOLESKY, 1990).
1.4 Adsorption mechanisms
The chemical structure and metal sorption mechanisms of biomass have been extensively studied. VEGLIO and BEOLCHINI (1997) classified the biosorption mechanisms into two main categories, according to their cell functionality, i.e., metabolism-dependant and non-metabolism-dependant. The metabolism-dependant mechanism involves transport across the cell membrane and a precipitation step (BRIERLEY, 1990; COSTA and LEITE, 1990), whereas the non-metabolism-dependant mechanism consists of precipitation (HOLAN et al., 1993; SCOTT and PALMER, 1990), physical adsorption (AKSU et al., 1992; ZHOU and KIFF, 1991), ion exchange (FRISS and MYERS-KEITH, 1986; MURALEEDHARAN and VENKOBACHAR, 1990) and complexation (CABRAL, 1992; TSEZOS and VOLESKY, 1981). Another way to classify the mechanism is based on the location where the extracted metal accumulates; for example, there is intracellular accumulation (transport across membrane), cell surface adsorption/precipitation (ion exchange, complexation, physical adsorption, precipitation) and extra-cellular accumulation/precipitation (VEGLIO and BOELCHINI, 1997).
1.5 Adsorption models
Various models have been used to evaluate the experimental data in order to identify the sorption mechanisms, i.e., chemisorption, physical adsorption or ion exchange. Some studies have compared the ion exchange model with the Langmuir adsorption model (DA COSTA and DE FRANÇA, 1996; FIGUEIRA et al., 2000). The Langmuir adsorption model assumes that only one type of adsorption site exists (i.e., all surface sites have equal activity) and that adsorption equilibrium is reached with the formation of a monolayer (STUMM and MORGAN, 1996). This model does not take into account the speciation of the metal in solution and, therefore, it applies only if the ionic strength, the pH and the ligand concentration are constants.
The ion exchange model has been found to give the best fit for metal sorption on algae biomass because the sorption is accompanied by the release of ions (e.g., Ca2+, Mg2+, Na+ and K+) (CRIST et al., 1994; KRATOCHVIL et al., 1998; KUYUCAK and VOLESKY, 1988; SCHIEWER and VOLESKY, 1995). Experiments have been carried out to study several system variables, such as the initial concentration (CRIST et al., 1994), the sorbent particle size (FISHER, 1985), and the solution pH (JANG et al., 1995; LEE and VOLESKY, 1997). Similarly, experimental data have been analyzed to determine the reaction kinetic order of a pore/solid phase diffusion mechanism (HO and MCKAY, 2000).
Other models used to describe various biosorption isotherms include the Freundlich model, a combination of the Langmuir and Freundlich models, the Radke and Prausnitz model, the Reddlich-Peterson model, the Brunauer (BET) model, and the Dubinin-Radushkevich model (VOLESKY, 2003). The «Ideal Absorbed Solution Theory» (IAST) and the «Surface Complexation» (SCM) models have also been used for solutions containing a mixture of metal ions (VOLESKY, 2003). Some other structured types of models taking into consideration the metal speciation in solution, the pH and the electrostatic attraction in solution have been proposed by SCHIEWER and WONG (1999) and YANG and VOLESKY (2000).
1.6 Metal recovery
The metal-laden biosorbent can be either eluted and reused or disposed of in a safe manner. In the former case, the biosorbent operates much like an ion exchange resin. Metals can be eluted using a specific solution (the eluant) to generate a small volume of a concentrated solution (the eluate). The choice of eluant depends on the metal ion to be eluted. Common and heavy metals (e.g., Cd, Co, Cu, Mn, Pb, Zn) are usually eluted with dilute mineral acids (e.g., HCl, H2SO4, and HNO3) or concentrated saline solutions (e.g. 0.5 M NaCl) (GARNHAM et al., 1992b). EDTA has been used in certain cases, but it is generally more expensive than mineral acids or saline solutions (HORIKOSHI et al., 1979). The adsorption of some noble metals, such as gold, silver and mercury, shows little or no dependence on pH and, consequently, these metals cannot be removed with dilute acid solutions (EDYVEAN et al., 1997). Thiourea or mercaptoethanol solutions have been used for recovering gold from biosorbents (DARNALL et al., 1986; GREENE and DARNALL, 1990). Similarly, sodium acetate solutions are effective for eluting copper and silver (HARRIS and RAMELO, 1990). Sodium carbonate has been used to desorb uranium from the algae Chlorella regularis (NAKAJIMA et al., 1982).
In general, biosorbents decompose and char at relatively low temperatures. Therefore, metal-laden biosorbents can be readily burned to produce an ash residue having a high metal concentration. This alternative may be economically viable for systems dealing with valuable metals and/or inexpensive biosorbents (GARNHAM, 1997). Alginic acid powder and calcium alginate beads have high affinity and capacity sorption for Fe(III), and the extraction of Fe(III) from acid synthetic solution is technically feasible (RIVEROS et al., 2001). Applied to acid mine drainage, the Fe(III) extraction would result in a significant reduction of the lime consumption and the volume of the neutralization sludge (RIVEROS, 2004). Once adsorbed, the Fe may be eluted and precipitated as hematite in order to obtain marketable and useful product (DUTRIZAC and RIVEROS, 1999).
1.7 Cost analyses
Preliminary costing evaluation for biosorption treatment options were conducted by ADERHOLD et al. (1996) and VOLESKY (1999). The results indicate that biosorption is a cost-effective technology. The cost-benefit analysis of any treatment option presents various difficulties, such as the lack of publicly available information on operating cost and the long-term impact of the treatment operation. This is particularly true for biotechnological processes. However, a comparative cost study was conducted for biosorptive processes with ion exchange and chemical precipitation (ECCLES, 1995). The selection of an effluent treatment system needs to comply with various criteria, such as compatibility with existing operations, cost effectiveness, flexibility to handle fluctuation in quality and quantity of effluent feed. The system should also be reliable, robust, selective and simple (ECCLES, 1995). Eccles compared the AMT-Bioclaim-process-based hard granular biomass, Bacillus subtilis (BRIERLEY et al., 1986) with the chemical precipitation method. The predicted results showed that the AMT-Bioclaim method could reduce the cost per gallon by over 50% when compared with a chemical treatment method. A second study was completed by the author to compare the Biofix process with chemical precipitation to treat acid mine drainage. The Biofix process consists of a mixture of biomass including bacteria, algae and fungi immobilized in polyethylene beads. Using the data from JEFFERS (1994), the acid mine drainage (AMD) treatment cost was 1.4 US$ per 1000 US gallons for the Biofix process and for conventional lime treatment, it was calculated the cost would correspond to 1.5 US$ (ECCLES, 1995). Table 5 summarizes the treatment options, along with their advantages and disadvantages.
Table 5
Treatment options for the removal of heavy metals (adapted from ADERHOLD et al., 1996).
Options de traitement pour l’enlèvement des métaux (adapté d’ADERHOLD et al., 1996).
Treatment options |
Advantages |
Disadvantages |
|---|---|---|
Lime precipitation |
Relatively inexpensive Bulk removal |
Non selective |
Ion exchange |
Multiple ion-exchange resins High specificity for heavy metal Several sorption and desorption cycles No sludge production Electrolysis allows metal recycling |
High capital and operating costs Economic of these process depends on energy price and the amount of electricity used per treated volume of solution |
|
Membrane processes Osmosis Reverse osmosis |
|
Very specialized application Limited flow rate Membrane instability in salt and acid conditions Prohibitive cost |
Adsorption processes |
Versatile, simple Selectively sorbed the sorbate Low cost technology |
|
2. Other Natural Sorbents
Several research papers have been published about the use of a variety of natural sorbents to remove metals from synthetic or industrial effluents. Table 6 shows different studies conducted on the utilization of natural sorbents for the removal of several metals (Ag, Al, As, Au, Ba, Bi, Ca, Cd, Co, Cr, Cu, Fe, Hg, Ir, Mg, Mn, Mo, Na, Ni, Os, Pb, Pd, Pt, Ra, Sb, St, Ti, Tl, V, Zn, Zr), lanthanides (Ce, Eu, La, Yb) and actinides (Th, U).
Table 6
Studies on the adsorption of different metals using natural adsorbents.
Études portant sur l’adsorption de différents métaux sur des adsorbants naturels.
Metals |
References |
|---|---|
Aluminium (Al) (III) |
Crist et al. (1994), Orhan and Büyükgüngör (1993), CUI et al. (2006) |
Antimony (Sb) (III) |
Coupal and Lalancette (1976), Masri and Friedman (1974) |
Arsenic (As) (II, V) |
Loukidouet al. (2003), Masri and Friedman (1974) |
Barium (Ba) (II) |
Cristet al. (1994), Smithet al. (1977) |
Bismuth (Bi) (III) |
Masri and Friedman (1974), Shimizu and Takada (1997) |
Cadmium (Cd) (II) |
Volesky and Prasetyo (1994), YU et al. (1999) |
Calcium (Ca) (II) |
Fiset et al. (2002), Fourest and Volesky (1997) |
Cerium (Ce) (III) |
Masri and Friedman (1974) |
Chromium (Cr)(III, VI) |
Baileyet al. (1992), FISHER et al. (1984) |
Cobalt (Co) (II) |
Flynnet al. (1980), Kuyucak and Volesky (1988) |
Copper (Cu) (I, II) |
MCKAY et al. (1999), YU et al. (1999), CUI et al. (2006) |
Europium (Eu) (III) |
Andreset al. (1993) |
Gold (Au) (III) |
Kuyucak and Volesky (1988), Nakajima (2003) |
Iridium (Ir) (IV) |
Ruizet al. (2003) |
Iron (Fe) (II, III) |
Fisetet al. (2002), Nassaret al. (2004), CUI et al. (2006) |
Lanthanum (La) (III) |
Bloom and McBride (1979), Cristet al. (1994) |
Lead (Pb) (II) |
Holan and Volesky (1994),YU et al.(1999), Murathan and Bütün (2006) |
Magnesium (Mg) (II) |
Crist et al. (1994), Fisetet al. (2002), CUI et al. (2006) |
Manganese (Mn) (II) |
Fiset et al. (2002), Nassaret al. (2004), CUI et al. (2006) |
Mercury (Hg) (I, II) |
FISHER et al. (1984), Viraraghavan and Kapoor (1994) |
Molybdenum (Mo) (VI) |
Guibal et al. (1999), Sakagushi et al. (1981) |
Nickel (Ni) (II) |
Flynnet al. (1980), Leuschet al. (1995) |
Osmium (Os) (IV) |
Ruizet al. (2003) |
Palladium (Pd) (II) |
Baba and Hirakawa (1992), Guibal et al. (2001) |
Platinum (Pt) (IV) |
Baba and Hirakawa (1992), Guibal et al. (2001) |
Radium (Ra) (II) |
TSEZOS (1997), TSEZOS and KELLER (1983) |
Silver (Ag) (I) |
FISHER et al. (1984), Flynnet al. (1980) |
Sodium (Na) (I) |
Fisetet al. (2002), Spintiet al. (1995) |
Strontium (Sr) (II) |
Shimizu and Takada (1997); Small et al. (1999) |
Technetium (Tc) (VII) |
Garnhamet al. (1992a, 1993b) |
Thallium (Tl) (I) |
Masri and Friedman (1974) |
Thorium (Th) (IV) |
Masri and Friedman (1974), Tsezos and Volesky (1981) |
Titanium (Ti) (IV) |
Parkash and Brown (1976) |
Uranium (U) (IV, VI) |
Guibal et al. (1994), Tsezos and Volesky (1982) |
Vanadium (V) (V) |
Guibal et al. (1994) |
Ytterbium (Yb) (III) |
Andres et al. (1993) |
Zinc (Zn) (II) |
Artola and Rigola (1992); Kuyucak and Volesky (1988) |
Zirconium (Zr) (IV) |
Garnhamet al. (1993a), Parkash and Brown (1976) |
2.1 Microorganisms
Many studies have been carried out on the utilization of dead or living microorganisms, including bacteria, yeasts, fungi, microalgae, cyanobacteria and activated sludge biomass for metal removal from solutions. Some examples of different microorganisms used for their metal adsorption capacity are presented in Table 7. The metal adsorption on the cell surface of non-living microorganisms usually involves different functional groups such as carboxyl, amino, hydroxyl, sulfhydryl, phosphate and sulfate groups (KAPOOR and VIRARAGHAVAN, 1997; URRUTIA, 1997).
Table 7
Some examples of the microorganisms studied for the metal recovery from solutions.
Exemples de microorganismes étudiés pour la récupération de métaux en solution.
Microorganisms |
References |
|---|---|
Bacteria |
|
Bacillussubtilis and spp. |
Beveridgeet al. (1982), Cotoraset al. (1992), GREEN-RUIZ (2006) |
Micrococcus spp. |
Cotoraset al. (1992), Loet al. (2003) |
Mycobacterium spp. |
Andres et al. (1993) |
Pseudomonas spp. |
Cabral (1992), Lopezet al. (2002), D’SOUZA et al. (2006) |
Streptomyces spp. |
Friss and Myers-Keith (1986), Mattuschka and Straube (1993) |
Zooglea ramigera |
Aksu et al. (1992), Norberg and Persson (1984) |
Yeasts |
|
Candida spp. |
Aksu and Donmez (2001) |
Candida utilis |
kujan et al. (2006) |
Saccharomyces cerevisiae |
Kuyucak and Volesky (1988), Volesky et al. (1993) |
Fungi |
|
Aspergillus niger |
Venkobachar (1990) |
Aureobasidium pullulans |
Gadd and De Rome (1988) |
Cladosporium resinae |
Gadd and De Rome (1988) |
Funalia trogii |
ARICAet al. (2004) |
Ganoderma lucidum |
Venkobachar (1990) |
Penicillium spp. |
Loukidouet al. (2003), Sayet al. (2003) |
Rhizopus arrhizus |
Fourest and Roux (1992), Tsezos and Volesky (1982) |
Trametes versicolor |
Bayramogluet al. (2003) |
Micro-algae |
|
Chlorella vulgaris and spp. |
Aksu and Acikel (1999), Mehtaet al. (2002) |
Chlamydomonas spp. |
Garnham et al. (1992a), Sakaguchiet al. (1981) |
Eudorina spp. |
Tien (2002) |
Euglena spp. |
Mannet al. (1988) |
Scenedesmus spp. |
Garnhamet al. (1993a), Sakaguchiet al. (1981) |
Synechocystis aquatilis |
ergene et al. (2006) |
Cyanobacteria |
|
Anabaena spp. |
Garnhamet al. (1993b), Tien (2002) |
Nostoc spp. |
Fernandez-Pinaset al. (1991), Hassett et al. (1981) |
Oscillatoria spp. |
Fisheret al. (1984), Tien (2002) |
Synechoccus spp. |
Garnham et al. (1993a, b), Sakaguchiet al. (1978) |
Other |
|
Activated sludge |
Artola and Rigola (1992), Hammainiet al. (2003) |
2.2 Forestry industry wastes
Forestry industry wastes including sawdust and tree barks, which are lignin/tannin-rich materials, have been also intensively studied for metal recovery from solutions (FISET et al., 2000; SEKI et al., 1997; VAISHYA and PRASAD, 1991). The polyhydroxy polyphenol groups of tannin are thought to be the active species in the metal adsorption (ion-exchange) process (VASQUEZ et al., 1994). Lignin extracted from black liquor, a waste product of the paper industry, has been considered for metal adsorption, specifically Hg, Pb and Zn (MASRI et al., 1974; SRIVASTAVA et al., 1994). Lignin (Figure 2) contains polar functional groups, such as alcohols, acids, aldehydes, ketones, phenol hydroxides and ethers, which have varying metal binding capabilities (BAILEY et al., 1999).
Figure 2
The chemical structure of lignin.
Structure chimique de la lignine.
2.3 Aquatic plants
Some aquatic plants (e.g.Ceratophyllum demersum, Lemna minor, Myriophyllum spicatum) have also been tested for phytoremediation or phytofiltration of metal-contaminated effluents (AXTELL et al., 2003; KESKINKAN et al., 2004 SCHNEIDER et al., 2001). Chemical modification and spectroscopic studies have shown that the cellular components include carboxyl, hydroxyl, sulfate, sulfhydryl, phosphate, amino, amide, imine, and imidazol moieties, which have metal binding properties and are, therefore, the functional groups in these plants (GARDEA-TORRESDEY et al., 2004).
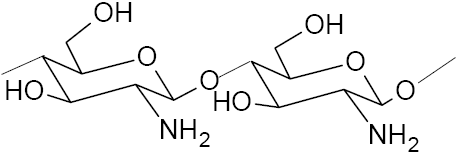
2.4 Chitin and chitosan
Various researchers have utilized chitin and chitosan for removing metal ions from effluents (MCKAY et al., 1989; HSIEN and RORRER, 1995). Chitin (Figure 3) is the second most abundant natural biopolymers after cellulose (BABEL et al., 2003). This natural biopolymer is widely found in the exoskeleton of shellfish and crustaceans (KIM and PARK, 2001). Chitosan (Figure 4) is produced by alkaline N-deacetylation of chitin. Crab shells or seafood processing waste sludge can also be used directly for metal adsorption without chitin extraction (KIM and PARK, 2001; LEE and DAVIS, 2001). The metal ions adsorption on chitosan mostly involved free amine groups. However, the binding ability of these sorbents for various metal ions is not directly proportional to the degree of free amine content (EDYVEAN et al., 1997).
Figure 3
The chemical structure of chitin.
Structure chimique de la chitine.
Figure 4
The chemical structure of chitosan.
Structure chimique du chitosan.
2.5 Peat moss
Peat moss, which is also very abundant in nature, has been intensively studied for water decontamination and particularly for the metal removal from waste streams (KERTMAN et al., 1993; SHARMA and FORSTER, 1993; VIRARAGHAVAN and RAO, 1993). Peat moss is a complex material, having lignin and cellulose as its major components. Both these components contain polar functional groups, such as carboxylic acids, phenol hydroxides, alcohols, aldehydes, ketones and ethers, which bind metal ions (BROWN et al., 2000. COUILLARD, 1994; WASE et al., 1997).
2.6 Agricultural wastes
Other types of natural sorbents proposed in the literature for metal retention include different agricultural wastes (e.g., tea/coffee and rice residues, fruit and vegetable peels, nut skins/husks). Some examples of these inexpensive and readily available materials are presented in Table 8. The polyhydroxy polyphenol groups, as well as, carboxylic and amino groups, found in these materials are involved in the metal adsorption (ion-exchange) process (MEUNIER et al., 2003b; RANDALL et al., 1974).
Table 8
Agricultural wastes studied for the metal recovery from solutions.
Déchets agricoles étudiés pour la récupération de métaux en solution.
Wastes |
References |
|---|---|
Banana pith and peels |
Annaduraiet al. (2003), Lowet al. (1995) |
Canola meal |
Al-Asheh and Duvnjak (1996) |
Carrot residues |
Nasernejadet al. (2005) |
Cassava fibre |
ABIAet al. (2006) |
Chicken feathers |
Al-Ashehet al. (2002) |
Cocoa shells |
Fiset et al. (2002), Meunieret al. (2003a, b, 2004) |
Coconut byproducts |
Randallet al. (1974), OFOMAJA and HO (2007), Mohan et al. (2006) |
Coffee residues |
Minamisawaet al. (2002) |
Corn cobs and roots |
Bosincoet al. (1996), Goldberg and Grieve (2003) |
Grape stalks |
Fiol et al. (2006), Escuderoet al. (2006) |
Indian mustard |
Crist et al. (2004) |
Modified wool |
Marshall and Champagne (1995) |
Nut and walnut shells |
Orhan and Büyükgüngör (1993) |
Olive mill residues |
Gharaibehet al. (1998), Veglioet al. (2003) |
Onion peels |
Kumar and Dara (1982) |
Orange peels |
Ajmalet al. (2000), Masriet al. (1974) |
Palm kernel fibre |
Ho and OFOMAJA (2006) |
Peanut skins |
Chamarthyet al. (2001), Randall et al. (1974) |
Petiolar felt-sheath of palm |
Iqbal and Saeed (2002) |
Rice byproducts |
Ajmal et al. (2003), Montanheret al. (2005) |
Sheep manure wastes |
Al-Rubet al. (2003) |
Sunflower seed peel |
Ozdemiret al. (2004) |
Tea leaves |
Orhan and Büyükgüngör (1993), Tee and Khan (1988) |
2.7 Miscellaneous sorbents
Finally, other natural sorbents studied include notably animal bones (BANAT et al., 2002), clays (e.g. bentonite, kaolinite, montmorillonite, wollastonite) (CELIS et al., 2000; PRADAS et al., 1994), human hair and teeth (HELAL et al., 2002; TAN et al., 1985), leaf mould (SHARMA and FORSTNER, 1994), sand (AWAN et al., 2003), metal oxides (Al, Fe, Mn – oxides) (BAILEY et al., 1992; TRIVEDI and AXE, 2001), vermicompost (PEREIRA and ARRUDA, 2003), xanthate (FLYNN et al., 1980; JAWED and TARE, 1991), and zeolites (e.g., clinoptilolite and chabazite) (GENÇ-FUHRMAN, 2007; LEPPERT, 1990; KALLO, 2001; OLIVEIRA et al., 2004).
2.8 Industrial applications
The majority of studies on metal adsorption on biosorbents have been carried out using synthetic solutions containing one or several metal ions (BLAIS et al., 2003; CRIST et al., 1994; MASRI and FRIEDMAN, 1974). However, many research papers have shown the efficiency of biosorbents for the removal of metal ions from industrial wastewaters and acid mine drainage solutions (MCGREGOR et al., 1998; UTGIKAR et al., 2000; ZOUMIS et al., 2000), landfill leachates (ABOLLINO et al., 2003; CECEN and GURSOY, 2001), tannery wastewaters (ALVES et al., 1993), electroplating effluents (AJMAL et al., 1996, 2000; ALVAREZ-AYUSO et al., 2003; LO et al., 2003), acid leachates from sewage sludge decontamination (FISET et al., 2002), acid leachates from soil decontamination (MEUNIER et al., 2004), and alkaline leachates from air pollution control residues from municipal solid waste incinerators (BLAIS et al., 2002a, BLAIS et al., 2002b).
Biosorption has proved to be effective for removing metal ions from contaminated solutions and effluents. The main advantages of biosorption over conventional treatment techniques include lower capital and operating costs, arising from the use of abundant and inexpensive natural products, and lower disposal cost of the spent adsorbents because of their biodegradable nature. However, industrial applications of biosorption are rare, and this situation has been attributed to the non-technical gaps involved in the commercialization of technological innovations (VOLESKY and NAJA, 2005). Furthermore, most biosorption studies have been conducted in batch systems, rather than in the continuous systems that are typical of industrial applications, such as fluidized bed and packed bed columns and continuous stirred tank reactors (MEHTA and GAUR, 2005). Despite these facts, VOLESKY and NAJA (2005) have identified some industrial operations which represent a big potential market for biosorption applications; these include electroplating and metal finishing, mining and ore processing, smelting, leather processing and printed circuit board manufacturing. However, as some of these sectors may be reluctant to novel biotechnology applications, biotechnology industries may have to share the risk with the industry. Therefore, biotechnology industry may have to develop partnership with industries in order to finance, build and operate the treatment plant or to provide a turnkey operating plant.
Conclusions
Biosorbents, especially those derived from seaweed and alginic acid, have attracted much interested in recent years as a source of inexpensive adsorbents for toxic metallic ions. Biosorbents are widely available in nature, can be readily produced under various forms, and are both non-toxic and biodegradable. These characteristics give them a definitive advantage over synthetic products for the removal of toxic metals from industrial effluents. The physical stability of biosorbents, especially in alkaline conditions continues to be a drawback and more research is needed in this area.
Parties annexes
References
- ABIA A.A., O.B. DIDI, E.D. ASUQUO (2006). Modeling of Cd2+ sorption kinetics from aqueous solutions onto some thiolated agricultural waste adsorbents. J. Appl. Sci., 6, 2549-2556.
- ABOLLINO O., M. ACETO, M. MALANDRINO, C. ARZANINI, E. MENTASTI (2003). Adsorption of heavy metals on Na-montmorillonite. Effect of pH and organic substances. Water Res., 37, 1619-1627.
- ADERHOLD D., C.J. WILLIAMS, R.G.J. EDYVEAN (1996). The removal of heavy-metal ions by seaweeds and their derivatives. Bioresource Technol., 58, 1-6.
- AJMAL M., R.A.K. RAO, R. AHMAD, J. AHMAD (2000). Adsorption studies on Citrus reticulata (Fruit peel of orange): removal and recovery of Ni(II) from electroplating wastewater. J. Hazard. Mater., 79, 117-131.
- AJMAL M., R.A.K. RAO, S. ANWAR, J. AHMAD, R. AHMAD (2003). Adsorption studies on rice husk: removal and recovery of Cd(II) from wastewater. Bioresource Technol., 86, 147-149.
- AJMAL M., R.A.K. RAO, B.A. SIDDIQUI (1996). Studies on the removal and recovery of Cr(VI) from electroplating wastes. Water Res., 30, 1478-1482.
- AKSU Z. and U. ACIKEL (1999). A single-staged bioseparation process for simultaneous removal of copper(II) and chromium(VI) by using C-vulgaris. Process Biochem., 34, 589-599.
- AKSU Z. and G. DONMEZ (2001). Comparison of copper(II) biosorptive properties of live and treated Candida sp. J. Environ. Sci. Health Part A. Toxic/Hazard. Subst. Environ. Eng., 36, 367-381.
- AKSU Z., G. EGRETLI, T. KUTSAL (1998). A comparative study of copper(II) biosorption on Ca-alginate, agarose and immobilized C. vulgaris in a packed-bed column. Process Biochem., 33, 393-400.
- AKSU Z., Y. SAG, T. KUTSAL (1992). The biosorption of copper(II) by C. vulgaris and Z. ramigera. Environ. Technol., 13, 579-586.
- AL-ASHEH S., F. BANAT, D. ALROUSAN (2002). Adsorption of copper, zinc and nickel ions from single and binary metal ion mixtures on to chicken feathers. Adsorption Sci. Technol., 20, 849-864.
- AL-ASHEH S. and Z. DUVNJAK (1996). Biosorption of chromium by canola meal. Water Qual. Res. J. Can., 31, 319-328.
- ALLOWAY B.J. and D.C. AYERS (1993). Chemical principles of environmental pollution. Blackie Academic & Professional, London, United Kingdom.
- AL-RUB F.A.A., M.H. EL-NAAS, F. BENYAHIA, I ASHOUR (2004). Biosorption of nickel on blank alginate beads, free and immobilized algal cells. Process Biochem., 39, 1767‑1773.
- AL-RUB F.A.A., M. KANDAH, N. ALDABAYBEH (2003). Competitive adsorption of nickel and cadmium on sheep manure wastes: Experimental and prediction studies. Sep. Sci. Technol., 38, 483-497.
- ALVAREZ-AYUSO E., A. GARCIA-SANCHEZ, X. QUEROL (2003). Purification of metal electroplating waste waters using zeolites. Water Res., 37, 4855-4862.
- ALVES M.M., C.G. GOZÁLEZ BEÇA, R. GUEDES DE CARVALHO, J.M. CASTANHEIRA, M.C. SOL PEREIRA, L.A.T. VASCONCELOS (1993). Chromium removal in tannery wastewaters “polishing” by Pinus sylverstris bark. Water Res., 27, 1333-1338.
- ANDRES Y., H.J. MACCORDICK, J.C. HUBERT (1993). Adsorption of several actinide (Th, U) and lanthanide (La, Eu, Yb) ions by Mycobacterium smegmatis. Appl. Microbiol. Biotechnol., 39, 413-417.
- ANNADURAI G., R.S. JUANG, D.J. LEE (2003). Adsorption of heavy metals from water using banana and orange peels. Water Sci. Technol., 47, 185-190.
- ARAÚJO M.M. and J.A. TEIXEIRA (1997). Trivalent chromium sorption on alginate beads. Int. Biodeterior Biodegradation, 40, 63-74.
- ARAVINDHAN R., B. MADHAN, J.R. RAO, B.U. NAIR (2004). Recovery and reuse of chromium from tannery wastewaters using Turbinaria ornata seaweed. J. Chem. Technol. Biotechnol., 79, 1251-1258.
- ARICA M.Y., G. BAYRAMOGLU, M. YILMAZ, S. BEKTAS, O. GENC (2004). Biosorption of Hg2+, Cd2+, and Zn2+ by Ca-alginate and immobilized wood-rotting fungus Funalia trogii. J. Hazard. Mater., 109, 191-199.
- ARTOLA A. and M. RIGOLA (1992). Selection of optimum biological sludge for zinc removal from wastewater by a biosorption process. Biotechnol. Lett., 14, 1199-1204.
- ATKINSON B.W., F. BUX, H.C. KASAN (1998). Considerations for application of biosorption technology to remediate metal-contaminated industrial effluents. Water SA, 24, 129-135.
- AWAN M.A., I.A. QAZI, I. KHALID (2003). Removal of heavy metals through adsorption using sand. J. Environ. Sci. China, 15, 413-416.
- AXTELL N.R., S.P.K. STERNBERG, K. CLAUSSEN (2003). Lead and nickel removal using Microspora and Lemna minor. Bioresource Technol., 89, 41-48.
- BABA Y. AND H. HIRAKAWA (1992). Selective adsorption of palladium(II), platinum(IV), and mercury(II) on a new chitosan derivative possessing pyridyl. Chem. Lett., 10, 1905-1908.
- BABEL S. and T.A. KURNIAWAN (2003). Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J. Hazard. Mater., 97, 219-243.
- BAILEY R.P., T. BENNETT, M.M. BENJAMIN (1992). Sorption onto and recovery of Cr(VI) using iron-oxide-coated sand. Water Sci. Technol., 26(5-6), 1239-1244.
- BAILEY S.E., T.J. OLIN, R.M. BRICKA, D. ARIAN (1999). A review of potentially low-cost sorbents for heavy metals. Water Res., 33, 2469-2479.
- BAJPAI, J., S R. HRIVASTAVA, A. K. BAJPAI (2004). Dynamic and equilibrium studies on adsorption of Cr(VI) ions onto binary bio-polymeric beads of cross linked alginate and gelatin. Colloids and Surfaces A: Physicochem. Eng. Aspects. 236. 81-90.
- BANAT F., S. ALASHEH, F. MOHAI (2002). Multi-metal sorption by spent animal bones. Sep. Sci. Technol., 37, 311‑327.
- BANKS C.J. (1997). Scavenging trace concentrations of metals. In: Biosorbents for metal ions. WASE J., FORSTER C. [Editors.], Taylor & Francis Ltd, London, United Kingdom, pp. 115-140.
- BAYRAMOGLU G., S. BEKTAS, M.Y. ARICA (2003). Biosorption of heavy metal ions on immobilized white-rot fungus Trametes versicolor. J. Hazard. Mater., 101, 285‑300.
- BEVERIDGE T.J., C.W. FORESBERG, R.J. DOYLE (1982). Major sites of metal binding in Bacillus licheniformis walls. J. Bacteriol., 150, 1438-1448.
- BLAIS J.F., S. DUFRESNE, G. MERCIER (1999). État du développement technologique en matière d’enlèvement des métaux des effluents industriels. Rev. Sci. Eau, 12, 687‑711.
- BLAIS J.F., MERCIER G., DURAND A. (2002b). Récupération du plomb et du zinc par adsorption sur tourbe lors de la décontamination de cendres volantes d’incinérateur de déchets municipaux. Environ. Technol., 23, 515-524.
- BLAIS J.F., S E. ALVANO, F. HAMMY, G. MERCIER (2002a). Comparaison de divers adsorbants naturels pour la récupération du plomb lors de la décontamination de chaux usées d’incinérateur de déchets municipaux. J. Environ. Eng. Sci., 1, 265-273.
- BLAIS J.F., S S. HEN, N. MEUNIER, R.D. TYAGI (2003). Comparison of different natural adsorbents for metal removal from acidic effluent. Environ. Technol. 24, 205‑215.
- BLOOM P.R., M.B. McBRIDE (1979). Metal ion binding and exchange with hydrogen ions in acid-washed peat. Soil Sci. Soc. Am. J., 43, 687-692.
- BORBA C.E., R. GUIRARDELLO, E.A. SILVA, M.T. VEIT, C.R.G. TAVARES (2006). Removal of nickel(II) ions from aqueous solution by biosorption in a fixed bed column: experimental and theoretical breakthrough curves. Biochem. Eng. J. 30, 184-191.
- BOSINCO S., J. ROUSSY, E. GUIBAL, P. LECLOIREC, (1996). Interaction mechanisms between hexavalent chromium and corncob. Environ. Technol., 17, 55-62.
- BRIERLEY C.L. (1990). Bioremediation of metal-contamined surfaces and groundwaters. Geomicrobiol. J., 8, 201-223.
- BRIERLEY I.A., G.M. GOYAK, C.L. BRIERLEY (1986). Considerations for commercial use of natural products for metals recovery. In: Immobilisation of ions by biosorption, H. Eccles & S.Hunt. Ellis Horwood (Editors), Chichester, pp.105-120.
- BROOKS C.S. (1991). Metal recovery from industrial wastes. Lewis Publishers (Editors), Boca Raton, Florida, UNITED STATES.
- BROWN P.A., S.A. GILL, S.J. ALLEN (2000). Metal removal from wastewater using peat. Water Res., 34, 3907-3916.
- CABRAL J.P.S. (1992). Selective binding of metal ions to Pseudomonas syringae cells. Microbios., 71, 47-53.
- CECEN F. and G. GURSOY (2001). Biosorption of heavy metals from landfill leachate onto activated sludge. J. Environ. Sci. Health Part A. Toxic/Hazard. Subst. Environ. Eng., 36, 987-998.
- CELIS R., M.C. HERMOSIN, J. CORNEJO (2000). Heavy metal adsorption by functionalized clays. Environ. Sci. Technol., 34, 4593-4599.
- CEPRIÁ G., L. IRIGOYEN, J.R. CASTILLO (2006). A microscale procedure to test the metal sorption properties of biomass sorbents: A time and reagents saving alternative to conventional methods. Microchim. Acta., 154, 287‑295.
- CHAISUKSANT Y. (2003). Biosorption of cadmium(II) and copper(II) by pretreated biomass of marine alga Gracilaria fisheri. Environ. Technol., 24, 1501-1508.
- CHAMARTHY S., C.W. SEO, W.E. MARSHALL (2001). Adsorption of selected toxic metals by modified peanut shells. J. Chem. Technol. Biotechnol., 76, 593-597.
- CHANG A.C., D.E. CROWLEY, A.L. PAGE (2003). Assessing bioavailability of metals in biosolids-treated soils. Water Environment Research Foundation, 97-REM-5, IWA Publishing, London, United Kingdom.
- CHEN D., Z. LEWANDOWSKI, F. ROE, P. SURAPANENI (1993). Diffusivity of Cu2+ in calcium alginate gel beads. Biotechnol. Bioeng., 41, 755-760.
- CHMIELEWSKI A.P., T.S. URBANSKI, W. MIGDAL (1997). Separation technologies for metals recovery from industrial wastes. Hydrometallurgy, 45, 333-344.
- COSSICH E.S., E.A. DA SILVA, C.R.G. TAVARES, L.C. FILHO, T.M.K. RAVAGNANI (2004). Biosorption of chromium (III) by biomass of seaweed Sargussum sp. in a fixed-bed column. Adsorption, 10, 129-138.
- COSTA A.C.A. and S.G.F. LEITE (1990). Cadmium and zinc biosorption by Chlorella homosphaera. Biotechnol Lett., 12, 941-944.
- COTORAS D., P. VIEDMA, L. CIFUENTES, A. MESTRE (1992). Sorption of metal ions by whole cells of Bacillus and Micrococcus. Environ. Technol., 13, 551-559.
- COUILLARD D. (1994). The use of peat in wastewater treatment. Water Res., 28, 1261-1274.
- COUPAL B. and J.M. LALANCETTE (1976). The treatment with waste waters with peat moss. Water Res., 10, 1071‑1076.
- CRIST R.H., J.R. MARTIN, D. CARR, J.R. WATSON, H.J. CLARKE (1994). Interaction of metals and protons with algae. 4. Ion exchange vs adsorption models and reassessment of scatchard plots; ion-exchange rates and equilibria compared with calcium alginate. Environ. Sci. Technol., 28, 1859-1866.
- CRIST R.H., J.R. MARTIN, D.L.R. CRIST (2004). Ion-exchange aspects of toxic metal uptake by Indian mustard. Int. J. Phytoremediation, 6, 85-94.
- CUI H., L.Y. LI, J. R. GRACE (2006). Exploration of remediation of acid rock drainage with clinoptilolite as sorbent in a slurry bubble column for both heavy metal capture and regeneration. Water Res., 40, 3359-3366.
- DA COSTA A.C.A. and F.P. DE FRANÇA (1996). Cadmium uptake by biosorbent seaweeds: adsorption isotherms and some process conditions. Sep. Sci. Technol., 31, 2373‑2393.
- DA COSTA A.C.A., L.M.S. DE MESQUITA, J. TORNOVSKY (1996). Batch and continuous heavy metal biosorption by a brown seaweed from a zinc-producing plant. Miner. Eng., 9, 811-824.
- DARNALL D.W., B. GREENE, M. HOSEA, R.A. McPHERSON, M. HENZL, M.D. ALEXANDER (1986). Recovery of metals from algae. In: Trace Metal Removal from Aqueous Solutions. THOMPSON R. (Editors), Litho Ltd, Whitstable, Kentucky, UNITED STATES, pp. 1-24.
- D’SOUZA S.F., P. SAR, S.K. KAZY, B. KUBAL (2006). Uranium sorption by Pseudomonas biomass immobilized in radiation polymerized polyacrylamide bio-beads. J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 41, 487-500.
- DUTRIZAC J.E. and P.A. RIVEROS (1999). Hematite precipitation from ferric chloride media at atmospheric pressure: A new approach to iron control and recycling. Proceedings of REWAS’99, A global symposium on recycling, waste treatment and clean technology, San Sebastian Spain, September 5-9. Gaballah I., Hager J. and Solozabal R. (Editors), TMS-Inasmet, 1, p. 663-673.
- ECCLES H. (1995). Removal of heavy metals from effluent streams - why select a biological process? Int. Biodeterioration Biodegradation, 5, 5-16.
- EDYVEAN R.G.J., C.J. WILLIAMS, M.W. WILSON, D. ADERHOLD (1997). Biosorption using unusual biomasses. In: Biosorbents for metal ions. WASE J., FORSTER C. (Editors), Taylor & Francis Ltd, London, United Kingdom, Chap. 8, pp. 165-812.
- ERGENE A., S. TAN, H. KATIRCIOGLU, Z. OKTEM (2006) Biosorption of copper(II) on immobilised Synechocystis aquatilis. Fresenius Environ. Bull. 15, 283-288.
- ESCUDERO C., N. FIOL, I. VILLAESCUSA (2006). Chromium sorption on grape stalks encapsulated in calcium alginate beads. Environ. Chem. Lett. 4, 239-242.
- FAO (2002). Food and agriculture organization of the United Nations. Yearbooks of fishery statistics production.
- FERNANDEZ-PINAS F., P. MATEO, I. BONILLA (1991). Binding of cadmium by cyanobacterial growth media : free ion concentration as a toxicity index to the cyano-bacteria Nostoc UAM 208. Arch. Environ. Contam. Toxicol., 21, 435-431.
- FIGUEIRA M.M., B. VOLESKY, K. AZARIAN, V.S.T. CIMINELLI (2000). Biosorption column performance with a metal mixture. Environ. Sci. Technol., 34, 4320-4326.
- FIOL N., C. ESCUDERO, J. POCH, I. VILLAESCUSA (2006). Preliminary studies on Cr(VI) removal from aqueous solution using grape stalk wastes encapsulated in calcium alginate beads in a packed bed up-flow column. React. Funct. Polym. 66, 795-807.
- FISET J.F., J.F. BLAIS, R. BEN CHEIKH, R.D. TYAGI (2000). Revue sur l’enlèvement des métaux des effluents par adsorption sur les sciures et les écorces de bois. Rev. Sci. Eau, 13, 323-347.
- FISET J.F., R.D. TYAGI, J.F. BLAIS (2002). Cocoa shells as adsorbent for metal recovery from acid effluent. Water Pollut. Res. J. Can., 37, 379-388.
- FISHER N.S. (1985). Bioaccumulation of metals by marine picoplankton. Marine Biol., 87, 137-142.
- FISHER N.S., M. BOHE, J.L. TEYSSIE (1984). Accumulation and toxicity of Cd, Zn, Ag and Hg in four marine phytoplankters. Marine Ecol. Prog. Series, 18, 201-213.
- FLYNN C.M. JR., T.G. CARNAHAN, R.E. LINDSTROM (1980). Adsorption of heavy metal ions by xanthated sawdust. Report of Investigations # 8427. United States Bureau of Mines.
- FOUREST E. and J.C. ROUX (1992). Heavy metal biosorption by fungal mycelial by-products: mechanism and influence of pH. Appl. Microbiol. Biotechnol., 37, 399-403.
- FOUREST E., and B. VOLESKY (1997). Alginate properties and heavy metal biosorption by marine algae. Appl. Biochem. Biotechnol., 67, 215-226.
- FRISS N. and P. MYERS-KEITH (1986). Biosorption of uranium and lead by Streptomyces longwoodensis. Biotechnol. Bioeng., 28, 21-28.
- GADD G.M. and L. DE ROME (1988). Biosorption of copper by fungal melanine. J. Appl. Microbiol. Biotechnol., 29, 610-617.
- GARDEA-TORRESDEY J.L., G. DELAROSA, J.R. PERALTA-VIDEA (2004). Use of phytofiltration technologies in the removal of heavy metals: A review. Pure Appl. Chem., 76, 801-813.
- GARNHAM G.W. (1997). The use of algae as metal biosorbents. In: Biosorbents for metal ions. WASE J., FORSTER C. (Editors), Taylor & Francis Ltd, London, United Kingdom, pp. 11-37.
- GARNHAM G.W., G.A. CODD, G.M. GADD (1992a). Uptake of technetium by freshwater green microalgae. J. Appl. Microbiol. Biotechnol., 37, 679-684.
- GARNHAM G.W., G.A. CODD, G.M. GADD (1992b). Accumulation of cobalt, zinc and manganese by the estuarine green microalgal Chlorella salina immobilised in alginate microbeads. Environ. Sci. Technol., 26, 1764‑1769.
- GARNHAM G.W., G.A. CODD, G.M. GADD (1993a). Accumulation of zirconium by microalgae and cyanobacteria. J. Appl. Microbiol. Biotechnol., 39, 666‑672.
- GARNHAM G.W., G.A. CODD, G.M. GADD (1993b). Accumulation of technetium by cyanobacteria. J. Appl. Phycol., 5, 307-315.
- GENÇ-FUHRMAN H., P.S. MIKKELSEN, A. LEDIN (2007). Simultaneous removal of As, Cd, Cr, Cu, Ni, and Zn from stormwater: Experimental comparison of 11 different sorbents. Water Res. 41, 591-602.
- GHARAIBEH S.H., W.Y. ABU-EL-SHA’R, M.M. AL-KOFAHI (1998). Removal of selected heavy metals from aqueous solutions using processes solid residue of olive mill products. Water Res., 32, 498-502.
- GOLDBERG S. and C.M. GRIEVE (2003). Boron adsorption by maize cell walls. Plant Soil, 251, 137-142.
- GONG R.M., Y. DING, H.J. LIU, Q.Y.,CHEN, Z.L. LIU (2005). Lead biosorption and desorption by intact and pretreated Spirulina maxima biomass. Chemosphere, 58, 125-130.
- GOTOH T., K. MATSUSHIMA, K.I. KIKUCHI (2004). Adsorption of Cu and Mn on covalently cross-linked alginate gel beads. Chemosphere, 55, 57-64.
- GREEN-RUIZ C. (2006). Mercury(II) removal from aqueous solutions by nonviable Bacillus sp. from a tropical estuary. Bioresour. Technol., 97, 1907-1911.
- GREENE B. and D.W. DARNALL (1990). Microbial oxygenic photoautotrophes (cyanobacteria and algae) for metal-ion binding. In: Microbial Mineral Recovery. EHRLICH H.L., BRIERLEY C.L. (Editors), McGraw-Hill, New-York, New-York, UNITED STATES, pp. 227-302.
- GUIBAL E., C. MILOT, J. ROUSSY (1999). Molybdate sorption by cross-linked chitosan beads: Dynamic studies. Water Environ. Res., 71, 10-17.
- GUIBAL E., M. RUIZ, T. VINCENT, A. SASTRE, R. NAVARROMENDOZA (2001). Platinum and palladium sorption on chitosan derivatives. Sep. Sci. Technol., 36, 1017-1040.
- GUIBAL E., I. SAUCEDO, M. JANSSON-CHARRIER, B. DELANGHE, P. LE CLOIREC (1994). Uranium and vanadium sorption by chitosan and derivatives. Water Sci. Technol., 30, 183-190.
- GUPTA V.K., A.K. SHRIVASTAVA, N. JAIN (2001). Biosorption of chromium(VI) from aqueous solutions by green algae Spirogyra species. Water Res., 35, 4079‑4085.
- HAMMAINI A., F. GONZALEZ, A. BALLESTER, M.L. BLAZQUEZ, J.A. MUNOZ (2003). Simultaneous uptake of metals by activated sludge. Min. Eng., 16, 723-729.
- HARRIS P.O. and G.J. RAMELOW (1990). Binding of metal ions by particulate biomass derived from Chlorella vulgaris and Scenedesmus quadricauda. Environ. Sci. Technol., 24, 220-234.
- HASSETT J.M., J.V. JENNET, J.E. SMITH (1981). Microplate technique for determining accumulation of metals by algae. Appl. Environ. Microbiol., 41, 1097-1106.
- HELAL A.A., G.A. ALIAN, H.A. MADBOULY (2002). Sorption of tin on human teeth. Health Phys., 82, 105‑108.
- HIRAI A., H. ODANI (1994). Sorption and transport of water vapor in alginic acid, sodium alginate, and alginate-cobalt complex films. J. Polymer Sci. Part B: Polymer Phys., 32, 2329‑2337.
- HO Y.S. and McKAY (2000). Batch sorber design using equilibrium and contact time data for the removal of lead. Water Air Soil Pollut., 124, 141-153.
- HO Y.S. and A.E. OFOMAJA (2006). Kinetic studies of copper ion adsorption on palm kernel fibre. J. Hazard. Mater. 137, 1796-1802.
- HOLAN Z.R. and B. VOLESKY (1994). Biosorption of lead and nickel by biomass of marine algae. Biotechnol. Bioeng., 43, 1001-1009.
- HOLAN Z.R., B. VOLESKY, I. PRASETYO (1993). Biosorption of cadmium by biomass of marine algae. Biotechnol. Bioeng., 41, 819-825.
- HORIKOSHI T., A. NAKAJIMA, T. SAKAGUSHI (1979). Studies on the accumulation of heavy metal elements in biological systems IV. Uptake of uranium by Chlorella regularis. Agric. Biol. Chem., 43, 617-623.
- HSIEN T.Y. and G.L. RORRER (1995). Effects of acylation and crosslinking on the material properties and cadmium ion adsorption capacity of porous chitosan beads. Sep. Sci. Technol., 30, 2455-2475.
- HUANG C., Y.C. CHUNG, M.R. LIOU (1996). Adsorption of Cu(II) and Ni(II) by pelletized biopolymer. J. Hazard. Mater., 45, 265-277.
- IBÁÑEZ J.P. and Y. UMETSU (2002). Potential of protanated alginate beads for heavy metals uptake. Hydrometallurgy, 64, 89-99.
- IBÁÑEZ J.P. and Y. UMETSU (2004). Uptake of trivalent chromium from aqueous solutions using protonated dry alginate beads. Hydrometallurgy, 72, 327-334.
- IQBAL M. and A. SAEED (2002). Removal of heavy metals from contaminated water by petiolar felt-sheath of palm. Environ. Technol., 23, 1091-1098.
- JANG L.K., D. NGUYEN, G.G. GEESEY (1999). Selectivity of alginate gel for Cu over Zn when acidic conditions prevail. Water Res., 33, 2817-2825.
- JANG L.K., D. NGUYEN, G.G. GEESY (1995). Effect of pH on the absorption of Cu(II) by alginate gel. Water Res., 29, 315-321.
- JAWED M. and V. TARE (1991). Application of starch xanthates for cadmium removal: a comparative evaluation. J. Appl. Polymer Sci., 42, 317-324.
- JEFFERS T.H., P.G. BENNETT, R.R. CORWIN (1994). Biosorption of metal contaminants using immobilised biomass-field studies. U.S. Bureau of Mines, R1 9461.
- JEON C., J.Y. PARK, Y.J. YOO (2002). Characteristics of metal removal using carboxylated alginic acid. Water Res., 36, 1814-1824.
- JEON C., Y.J. YOO, W.H. HOELL (2005). Environmental effects and desorption characteristics on heavy metal removal using carboxylated alginic acid. Bioresource Technol., 96, 15-19.
- KAEWSARN P. (2002). Biosorption of copper(II) from aqueous solutions by pre-treated biomass of marine algae Padina sp. Chemosphere, 47, 1081-1085.
- KAEWSARN P., Q.M. YU, W.D. MA (2001). Interference of co-ions in biosorption of Cu2+ by biosorbent from marine alga Durvillaea potatorum. Environ. Eng. Sci., 18, 99‑104.
- KAEWSARN P. and Q.M. YU (2001). Cadmium(II) removal from aqueous solutions by pre-treated biomass of marine alga Padina sp. Environ. Pollut., 112, 209-213.
- KALLO D. (2001). Applications of natural zeolites in water and wastewater treatment. In: Natural Zeolites: Occurrence, Properties, Applications (Reviews in Mineralogy and Geochemistry, Vol. 45) BISH D.L., MING D.W., [Ed.], Mineralogical Society of America - The Geochemical Society, Washington, DC, U.S.A, pp. 519-550.
- KAPOOR A. and T. VIRARAGHAVAN (1997). Fungi as biosorbents. In: Biosorbents for metal ions. WASE J., FORSTER C. (Editors), Taylor & Francis Ltd, London, United Kingdom, pp. 67-85.
- KARAGUNDUZ A., Y. KAYA, B. KESKINLER, S. ONCEL (2006). Influence of surfactant entrapment to dried alginate beads on sorption and removal of Cu2+ ions. J. Hazard. Mater. 131, 79-83.
- KERTMAN S.V., G.M. KERTMAN, Z.S. CHIBRIKOVA (1993). Peat as a heavy-metal sorbent. J. Appl. Chem. U.S.S.R., 66, 465-466.
- KESKINKAN O., M.Z.L. GOKSU, M. BASIBUYUK, C.F. FORSTER (2004). Heavy metal adsorption properties of a submerged aquatic plant (Ceratophyllum demersum). Bioresource Technol., 92(2), 197-200.
- KHANI M. H., KESHTKAR A. R., MEYSAMI B., ZAREA M. F., JALALI R. (2006) Biosorption of uranium from aqueous solutions by non living biomass of marine algae Cystoseira indica. Electronic J. Biotechnol., 9, 100-106.
- KIM D.S. and B.Y. PARK (2001). Effects on the removal of Pb2+ from aqueous solution by crab shell. J. Chem. Technol. Biotechnol., 76, 1179-1184.
- KLIMMEK S., H.J. STAN, A. WILKE, G. BUNKE, R. BUCHHOLZ (2001). Comparative analysis of the biosorption of cadmium, lead, nickel, and zinc by algae. Environ. Sci. Technol., 35, 4283-4288.
- KRATOCHVIL D., P. PIMENTEL, B. VOLESKY (1998). Removal of trivalent and hexavalent chromium by seaweed biosorbent. Environ. Sci. technol., 32, 2693-2698.
- KUJAN P., A. PRELL, H. ŠAFÁR, M. SOBOTKA, T. REZANKA, P. HOLLER (2006). Use of the industrial yeast Candida utilis for cadmium sorption. Folia Microbiol., 51, 257-260.
- KUMAR P. and S.S. DARA (1982). Utilization of agricultural wastes for decontaminating industrial/domestic wastewaters from toxic metals. Agr. Wastes, 4, 213-223.
- KUMAR Y.P., P. KING, V.S.R.K. PRASAD (2006). Removal of copper from aqueous solution using Ulva fasciata sp. - A marine green algae. J. Hazard. Mater. 137, 367-373.
- KUYUCAK N. and B. VOLESKY (1988). Biosorbents for the recovery of metals from industrial solutions. Biotechnol. Lett., 10, 137-142.
- LAU T.C., P.O. ANG, P.K. WONG (2003). Development of seaweed biomass as a biosorbent for metal ions. Water Sci. Technol., 47, 49-54.
- LEE H.S. and B. VOLESKY (1997). Interaction of light metals and protons with seaweed biosorbent. Water Res., 31, 3082-3088.
- LEE S.M. and A.P. DAVIS (2001). Removal of Cu(II) and Cd(II) from aqueous solution by seafood processing waste sludge. Water Res., 35, 534-540.
- LEPPERT D. (1990). Heavy metal sorption with clinoptilolite zeolite: alternatives for treating contaminated soil and water. Mining Eng., 42, 604-608.
- LEUSCH A., Z.R. HOLAN, B. VOLESKY (1995). Biosorption of heavy metals (Cd, Cu, Ni, Pb, Zn) by chemically-reinforced biomass of marine algae. J. Chem. Technol. Biotechnol., 62, 279-288.
- LIPPMANN M. (2000). Environmental toxicants. Human exposures and their health effects. Wiley Interscience, New York, New York, United States, 987 pages.
- LO W., H. CHUA, M.F. WONG, P. YU (2003). Bacterial biosorbent for removing and recovering copper from electroplating effluents. Water Sci. Technol., 47, 251-256.
- LODEIRO P., J.L. BARRIADA, R. HERRERO, M.E. SASTRE DE VICENTE (2006). The marine macroalga Cystoseira baccata as biosorbent for cadmium(II) and lead(II) removal: kinetic and equilibrium studies. Environ. Pollut. 142, 264‑273.
- LODEIRO P., B. CORDERO, Z. GRILLE, R. HERRERO, M.E. SASTRE DE VIVENTO (2004). Physicochemical studies of cadmium(II) biosorption by the invasive alga in Europe Sargassum muticum. Biotechnol. Bioeng, 88, 237‑247.
- LOPEZ A., N. LAZARO, S. MORALES, A.M. MARQUES (2002). Nickel biosorption by free and immobilized cells of Pseudomonas fluorescens 4F39: A comparative study. Water Air Soil Pollut., 135, 157-172.
- LOUKIDOU M.X., K.A. MATIS, A.I. ZOUBOULIS, M. LIAKOPOULOU-KYRIAKIDOU (2003). Removal of As(V) from wastewaters by chemically modified fungal biomass. Water Res., 37(18), 4544-4552.
- LOW K.S., C.K. LEE, A.C. LEO (1995). Removal of metals from electroplating wastes using banana pith. Bioresource Technol., 51, 227-231.
- LU Y., E. WILKINS (1996). Heavy metal removal by caustic-treated yeast immobilized in alginate. J. Hazard. Mater., 49, 165-179.
- LUO F., Y. LIU, X. LI, Z. XUAN, J. MA (2006). Biosorption of lead ion by chemcially-modified biomass of marine algae Laminaria japonica. Chemsophere, 64, 1122-1127.
- MANN H., W.S. FYFE, R. KERRICH (1988). The chemical content of algae and waters: bioconcentration. Toxicity Assessment, 3, 1-16.
- MARSHALL W.E. and E.T. CHAMPAGNE (1995). Agricultural byproducts as adsorbents for metal ions in laboratory prepared solutions and in manufacturing wastewaters. J. Environ. Sci. Health, A30, 241-261.
- MASRI M.S. and M. FRIEDMAN (1974). Effect of chemical modification of wool on metal ion binding. J. Appl. Polymer Sci., 18, 2367-2377.
- MASRI M.S., F.W. REUTER, M. FRIEDMAN (1974). Binding of metal cations by natural substances. J. Appl. Polymer Sci., 18, 675-681.
- MATHEICKAL J.T., J. FELTHAM, Q. YU (1997). Cu(II) binding by marine alga Ecklonia radiata biomaterial. Environ. Technol., 18, 25-34.
- MATHEICKAL J.T. and Q. YU (1997). Biosorption of lead(II) from aqueous solutions by Phellinus badius. Miner. Eng., 10, 947-957.
- MATTUSCHKA B. and G. STRAUBE (1993). Biosorption of metals by a waste biomass. J. Chem. Technol., 58, 57‑63.
- McGREGOR R.G., D.W. BLOWES, J.L. JAMBOR, W.D. ROBERTSON (1998). Mobilization and attenuation of heavy metals within a nickel mine tailings impoundment near Sudbury, Ontario, Canada. Environ. Geol., 36, 305‑319.
- McKAY G., H.S. BLAIR, A. FINDON (1989). Equilibrium studies for the sorption of metal ions onto chitosan. Ind. J. Chem. A, 28, 356-360.
- MCKAY G., Y.S. HO, J.C.Y. NG (1999). Biosorption of copper from waste waters. A review. Sep. Purif. Methods, 28, 87-125.
- MEHTA S.K. and J.P. GAUR (2005). Use of algae for removing heavy metal ions from wastewater: progress and prospects. Crit. Rev. Biotechnol., 25, 113-152.
- MEHTA S.K., S A. INGH, J.P. GAUR (2002). Kinetics of adsorption and uptake of Cu2+ by Chlorella vulgaris: Influence of pH, temperature, culture age, and cations. J. Environ. Sci. Health Part A. Toxic/Hazard. Subst. Environ. Eng., 37, 399-414.
- MEUNIER N., J.F. BLAIS, R.D. TYAGI (2004). Removal of heavy metals from acid soil leachate using cocoa shells in a counter-current sorption process. Hydrometallurgy, 73, 225-235.
- MEUNIER N., J. LAROULANDIE, J.F. BLAIS, R.D. TYAGI (2003a). Cocoa shells for heavy metal removal from acidic solutions. Bioresource Technol., 90, 255-263.
- MEUNIER N., J. LAROULANDIE, J.F. BLAIS, R.D. TYAGI (2003b). Lead removal from acidic solutions by sorption on cocoa shells: effect of some parameters. J. Environ. Eng. Div. ASCE, 129, 693-698.
- MIN J.H. and J.G. HERING 1998. Arsenate sorption by Fe(III)-doped alginate gels. Water Res., 32, 1544-1552.
- MINAMISAWA M., S. NAKAJIMA, Y. MITSUE, K. MIYAZAWA, K. UENO, A. HATORI, S. MIYAJIMA, M. HOSHINO, S. YOSHIDA, N. TAKAI (2002). Removal of copper(II) and cadmium(II) in water by use of roasted coffee beans. Nippon Kagaku Kaishi, 3, 459-461.
- MISRA V. and S.D. PANDEY (2001). Removal of arsenic from aqueous solutions by adsorption method using calcium alginate beads containing humic acid. J. Ecophysiol. Occup. Health, 1, 295-302.
- MOHAN D., K.P. SINGH, V.K. SINGH (2006). Trivalent chromium removal from wastewater using low cost activated carbon derived from agricultural waste material and activated carbon. J. Hazard. Mater. 135, 280-295.
- MONTANHER S.F., E.A. OLIVEIRA, M.C. ROLLEMBERG (2005). Removal of metal ions from aqueous solutions by sorption onto rice bran. J. Hazard. Mater., 117, 207-211.
- MURALEEDHARAN T.R. and C. VENKOBACHAR (1990). Mechanism of biosorption of copper(II) by Ganoderma lucidum. Biotechnol. Bioeng., 35, 320-325.
- MURATHAN A.S. and M. BÜTÜN (2006). Removal of lead ions from dilute aqueous solution in packed columns by using natural fruit shell through adsorption. Fresenius Environ. Bull., 15, 1491-1498.
- NAJA G. and B. VOLESKY (2006). Multi-metal biosorption in a fixed-bed flow-through column. Colloids and Surf., A, 281, 194-201.
- NAKAJIMA A. (2003). Accumulation of gold by micro-organisms. World J. Microbiol. Biotechnol., 19, 369-374.
- NAKAJIMA A., T. HORIKOSHI, T. SAKAGUCHI (1982). Recovery of uranium by immobilized microorganisms. Eur. J. Microbiol. Biotechnol., 16, 88-91.
- NASERNEJAD B., T.E. ZADEH, B.B. POUR, M.E. BYGI, A. ZAMANI (2005). Camparison for biosorption modeling of heavy metals (Cr(III), Cu(II), Zn(II)) adsorption from wastewater by carrot residues. Process Biochem., 40, 1319‑1322.
- NASSAR M.M., K.T. EWIDA, E.E. EBRAHIEM, Y.H. MAGDY, M.H. MHEAEDI (2004). Adsorption of iron and manganese using low cost materials as adsorbents. J. Environ. Sci. Health Part A. Toxic/Hazard. Subst. Environ. Eng., 39, 421-434.
- NESTLE N. and R. KIMMICH (1996). NMR Microscopy of heavy metal absorption in calcium alginate beads. Appl. Biochem. Biotechnol., 56, 9-17.
- NGOMSIK A.F., A. BEE, J.M. SIAUGUE, V. CABUIL, G. COTE (2006). Nickel adsorption by magnetic alginate microcapsules containing an extractant. Water Res. 40, 1848-1856.
- NORBERG A.B. and H. PERSSON (1984). Accumulation of heavy metal ions by Zooglea ramigera. Biotechnol. Bioeng., 26, 239-246.
- OFER R., A. YERACHMIEL, Y. SHMUEL (2003). Marine macroalgae as bisorbents for cadmium and nickel in water. Water Environ. Res., 75, 246-253.
- OFOMAJA A.E. and Y.S. LO (2007). Effect of pH on cadmium biosorption by coconut copra meal. J. Hazard. Mater. 139, 356-362.
- OLIVEIRA L.C.A., D.I. PETKOWICZ, A. SMANIOTTO, S.B.C. PERGHER (2004). Magnetic zeolites: a new adsorbent for removal of metallic contaminants from water. Water Res., 38, 3699-3704.
- ORHAN Y. and H. BÜYÜKGÜNGÖR (1993). The removal of heavy metals by using agricultural wastes. Water Sci. Technol., 28, 247-255.
- OZDEMIR G., N. CEYHAN, E. MANAV (2005). Utilization un alginate beads for Cu(II) and Ni(II) adsorption of an exopolysaccharide produced by Chryseomonas luteola Tem05. World J. Microbiol. Biotechnol. 21, 163-167.
- OZDEMIR M., O. SAHIN, E. GULER (2004). Removal of Pb(II) ions from water by sunflower seed peel. Fresenius Environ. Bull., 13, 524-531.
- PAPAGEORGIOU S.K., F.K. KATSAROS, E.P. KOUVELOS, J.W. NOLAN, H. LE DEIT, N.K. KANELLOPOULOS (2006). Heavy metal sorption by calcium alginate beads from Laminaria digitata. J. Hazard. Mater. 137, 1765‑1772.
- PARK D., Y.S. YUN, H.Y. CHO, J.M. PARK (2004). Chromium biosorption by thermally treated biomass of the brown seaweed, Ecklonia sp. Ind. Eng. Chem. Res., 43, 8226-8232.
- PARK H.G. and M.Y. CHAE (2004). Novel type of alginate gel-based adsorbents for heavy metal removal. J. Chem. Technol. Biotechnol., 79, 1080-1083.
- PARKASH A.S. and R.A.S. BROWN (1976). Use of peat and coal for recovering zirconium from solution. Can. Min. Metallurgical Bull., 69, 59-64.
- PATTERSON J.W. (1989). Industrial wastes reduction. Environ. Sci. Technol., 23, 1032-1038.
- PAVASANT P., R. APIRATIKUL, V. SUNGKHUM, P. SUTHIPARINYANONT, S. WATTANACHIRA, T. F. MARHABA (2006). Biosorption of Cu2+, Cd2+, Pb2+, and Zn2+ using dried marine green macroalga Caulerpa lentillifera. Bioresour. Technol. 97, 2321-2329.
- PEREIRA M.G. and M.A.Z. ARRUDA (2003). Vermicompost as a natural adsorbent material: Characterization and potentialities for cadmium adsorption. J. Braz. Chem. Soc., 14, 39-47.
- PRADAS E.G., M.V. SÁNCHEZ, F.C. CRUZ, M.S. VICIANA, M.F. PÉREZ (1994). Adsorption of cadmium and zinc from aqueous solution on natural and activated bentonite. J. Chem. Technol. Biotechnol., 59, 289-295.
- PRASHER S.O., M. BEAUGEARD, J. HAWARI, P. BERA, R.M. PATEL, S.H. KIM (2004). Biosorption of heavy metals by red algae (Palmaria palmata). Environ. Technol., 25, 1097-1106.
- RANDALL J.M., R.L. BERMANN, V. GARRETT, A.C. JR. WAISS (1974). Use of bark to remove heavy metal ions from waste solutions. Forest Prod. J., 24, 80-84.
- RENN, D. (1997). Biotechnology and the red seaweed polysaccharide industry: Status, needs and prospects. Trends Biotechnol., 15, 9-14.
- RIVEROS P.A. (2004). Iron removal from effluents to reduce sludge formation and CO2 emissions. European Commission-CANMET Joint Workshop on Clean Production Technologies, ADJEMIAN A., DUTRIZAC J., NÉGRÉ P., YURRAMENDI L. (Editors), Madrid, Spain, Sep 29 ‑Oct 2, 2004., Inasmet, San Sebastian, Spain, pp. 253-266.
- RIVEROS P.A., J.D. KAWAJA, D. GOULD, D. KOREN (2001). The extraction of Fe(III) from acid mine drainage using a biosorbent derived from seaweed. CANMET Report MMSL-INT 2001-005 (TR), Ottawa, Ontario, Canada.
- RUIZ M., A. SASTRE, E. GUIBAL (2003). Osmium and iridium sorption on chitosan derivatives. Solvent Extr. Ion Exc., 21, 307-329.
- SAKAGUCHI T., T. HORIKOSHI, A. NAKAJIMA (1978). Uptake of uranium from sea water by microalgae. J. Ferment. Technol., 56, 561-565.
- SAKAGUCHI T., A. NAKAJIMA, T. HORIKOSHI (1981). Studies on the accumulation of heavy metal elements in biological systems XVII. Accumulation of molybdenum by green microalgae. Eur. J. Appl. Microbiol. Biotechnol., 12, 84-89.
- SAY R., N. YIMAZ, A. DENIZLI (2003). Removal of heavy metal ions using the fungus Penicillium canescens. Ads. Sci. Technol., 21, 643-650.
- SCHIEWER S. and VOLESKY (1995). Modeling of the proton-metal ion exchange. Environ. Sci. technol., 29, 2921-2927.
- SCHIEWER S. and M.H. WONG (1999). Metal binding stoichiometry and isotherm choice in biosorption. Environ. Sci. Technol., 33, 3821-3828.
- SCHNEIDER I.A.H., J. RUBIO, R.W. SMITH (2001). Biosorption of metals onto plant biomass: exchange adsorption or surface precipitation? Int. J. Miner. Proc., 62, 111-120.
- SCOTT J.A. and S.J. PALMER (1990). Sites of cadmium uptake in bacteria used for biosorption. Appl. Microbiol. Biotechnol., 33, 221-225.
- SEKI H. and A. SUZUKI (1996). Adsorption of lead ions on composite biopolymer adsorbent. Ind. Eng. Res., 35, 1378‑1382.
- SEKI K., N. SAITO, M. AOYAMA (1997). Removal of heavy metal ions from solutions by coniferous barks. Wood Sci. Technol., 31, 441-447.
- SENTHILKUMAR R., K. VIJAYARAGHAVAN, M. THILAKAVATHI, P.V.R. IYER, M. VELAN (2006). Seaweeds for the remediation of wastewaters contaminated with zinc(II) ions. J. Hazard. Mater., 136, 791-799.
- SHARMA D.C. and C.F. FORSTER (1993). Removal of hexavalent chromium using Sphagnum moss peat. Water Res., 27, 1201-1208.
- SHARMA D.C. and C.F. FORSTER (1994). The treatment of chromium wastewaters using the sorptive potential of leaf mould. Bioresource Technol., 49, 31-40.
- SHIMIZU T. and A. TAKADA (1997). Preparation of Bi-based superconducting fiber by metal biosorption of Na-Alginate. Polymer Gels Networks, 5, 267-283.
- SINGHAL R.K., S. JOSHI, K. TIRUMALESH, R.P. GURG (2004). Reduction of uranium concentration in well water by Chlorella (Chlorella pyrendoidosa) a fresh water algae immobilized in calcium alginate. J. Radioanal. Nuclear Chem., 261, 73-78.
- SMALL T.D., L.A. WARREN, E.E. RODEN, F.G. FERRIS (1999). Sorption of strontium by bacteria, Fe(III) oxide, and bacteria-Fe(III) oxide composites. Environ. Sci. Technol., 33, 4465-4470.
- SMITH E.F., P. MACCARTHY, T.C. YU, H.B. JR. MARK (1977). Sulfuric acid treatment of peat for cation exchange. J. Water Pollut. Control Fed., April, 633-638.
- SPINTI M., H. ZHUANG, E.M. TRUJILLO (1995). Evaluation of immobilized biomass beads removing heavy metals from wastewaters. Water Environ. Res., 67, 943‑952.
- SRIVASTAVA S.K., A.K. SINGH, A. SHARMA (1994). Studies on the uptake of lead and zinc by lignin obtained from black liquor – a paper industry waste material. Environ. Technol., 15, 353-361.
- STUMM W. and J.J. MORGAN (1996). Aquatic chemistry chemical equilibria and rates in natural waters. SCHNOOR J.L., ZEHNDER A. (Editors], John Wiley and Sons, New York, New York, UNITED STATES, pp. 519-526.
- TAN T.C., C.K. CHIA, C.K. TEO (1985). Uptake of metal ions by chemically treated human hair. Water Res., 19, 157‑162.
- TEE T.W. and R.M. KHAN (1988). Removal of lead, cadmium and zinc by waste tea leaves. Environ. Technol. Lett., 9, 1223-1232.
- TIEN C.J. (2002). Biosorption of metal ions by freshwater algae with different surface characteristics. Proc. Biochem., 38, 605-613.
- TOTI U.S. and T.M. AMINABHAVI (2002). Pervaporation separation of water-isopropyl alcohol mixtures with blend membranes of sodium alginate and poly(acrylamide)-grafted guar gum. J. Appl. Polymer Sci., 85, 2014-2024.
- TRIVEDI P. and L. AXE (2001). Predicting divalent metal sorption to hydrous Al, Fe, and Mn oxides. Environ. Sci. Technol., 35, 1779-1784.
- TSEZOS M. (1997). Biosorption of lanthanides, actinides and related materials. In: Biosorbents for metal ions. WASE J., FORSTER C. [Ed.], Taylor & Francis Ltd, London, United Kingdom, pp. 87-113.
- TSEZOS M. and D. KELLER (1983). Adsorption of radium-226 by biological origin adsorbents. Biotechnol. Bioeng., 25, 201-215.
- TSEZOS M. and B. VOLESKY (1981). Biosorption of uranium and thorium. Biotechnol. Bioeng., 23, 583-604.
- TSEZOS M. and B. VOLESKY (1982). The mechanism of uranium biosorption by Rhizopus arrhizus. Biotechnol. Bioeng., 24, 385-401.
- TSUI M.T.K., K.C. CHEUNG, N.F.Y. TAM, M.H. WONG (2006). A comparative study on metal sorption by brown seaweed. Chemosphere, 65, 51-57.
- URRUTIA M.M. (1997). General bacterial sorption processes. In: Biosorbents for metal ions. WASE J., FORSTER C. [Ed.], Taylor & Francis Ltd, London, United Kingdom, pp. 39-66.
- UTGIKAR V., B.Y. CHEN, H.H. TABAK, D.F. BISHOP, R. GOVIND (2000). Treatment of acid mine drainage: I. Equilibrium biosorption of zinc and copper on non-viable activated sludge. Int. Biodeterioration Biodegradation, 46, 19-28.
- VAISHYA R.C. and S.C. PRASAD (1991). Adsoprtion of copper(II) on sawdust. Indian J. Environ. Protection, 11, 284-289.
- VÁZQUEZ G., G. ANTORRENA, J. GONZÁLEZ, M.D. DOVAL (1994). Adsorption of heavy metal ions by chemically modified Pinus Pinaster bark. Bioresource Technol., 48, 251-255.
- VEGLIO F. and F. BEOLCHINI (1997). Removal of metals by biosorption: a review. Hydrometallurgy, 44, 301-316.
- VEGLIO F., A. ESPOSITO, A.P. REVERBERI (2002). Copper adsorption on calcium alginate beads: equilibrium pH-related models. Hydrometallurgy, 65, 43-57.
- VEGLIO, F., F. BEOLCHINI, M. PRISCIANDARO (2003). Sorption of copper by olive mill residues. Water Res.. 37, 4895-4903.
- VENKOBACHAR C. (1990) Metal removal by waste biomass to upgrade wastewater treatment plants. Water Sci. Technol., 22, 319-320.
- VIRARAGHAVAN T. and A. KAPOOR (1994). Adsorption of mercury from wastewater by bentonite. Appl. Clay Sci., 9, 31-49.
- VIRARAGHAVAN T. and G.A.K. RAO (1993). Adsorption of cadmium and chromium from wastewater by peat. Int. J. Environ. Studies, 44, 9-27.
- VOLESKY B. (1990). Biosorption of heavy metals. CRC Press, Boca Raton, Florida, UNITED STATES.
- VOLESKY B. (1994). Advances in biosorption of metals: selection of biomass type. FEMS Microbiol. Rev., 14, 291‑302.
- VOLESKY B. (1999). Biosorption for the next century. In: Biohydrometallurgy and the Environment Toward the Mining of the 21st Century, Internat. Biohydrometallurgy Symposium Proceedings, volume B, BALLESTER, A. and AMILS, R. [Ed.] Elsevier Sciences, Amsterdam, The Netherlands, pp.161-170.
- VOLESKY B. (2003). Biosorption process simulation tools. Hydrometallurgy, 71, 179-190.
- VOLESKY B. H. MAY, Z.R. HOLAN (1993). Cadmium biosorption by Saccharomyces cerevisiae. Biotechnol. Bioeng., 41, 826-829.
- VOLESKY B. and Z.R. HOLAN (1995). Biosorption of heavy metals. Biotechnol. Prog., 11, 235-250.
- VOLESKY B. and G. NAJA (2005). Biosorption: Application Strategies. In: Proceedings of the 16th Internat. Biotechnol. Symp, IBS-Compress Co., Cape Town, South Africa, pp. 531-542.
- VOLESKY B. and I. PRASETYO (1994). Cadmium removal in a biosorption column. Biotechnol. Bioeng., 43, 1010‑1015.
- WANG L.S., W. HAI-SUO, Z. AI-QIANG (2004). Immobilization study of biosorption of heavy metal ions onto activated sludge. J. Environ. Sci., 16, 640-645.
- WASE D.A.J. and C.F. FORSTER (1997). Biosorbents for metal ions. Taylor & Francis Ltd (Editors), London, United Kingdom, 238 pages.
- WASE D.A.J., C.F. FORSTER, Y.S. HO (1997). Low-cost biosorbents: batch processes. In: Biosorbents for metal ions. WASE J., FORSTER C. (Editors), Taylor & Francis Ltd, London, United Kingdom, pp. 141-163.
- WATTS R.J. (1998). Hazardous wastes: sources, pathways, receptors. John Wiley & Sons, (Editors) New York, New York, United States, 764 pages.
- WILLIAMS C.J. and G.J. EDYVEAN (1997). Ion exchange in nickel biosorption by seaweed materials. Biotechnol. Prog., 13, 424-428.
- WILSON M.W. and R.G. EDYVEAN (1994). Biosorption for the removal of heavy metals from industrial wastewaters. Institution of Chemical Engineers Symposium Series 1994. Environ. Biotechnol., 89-91.
- YANG J. and B. VOLESKY (2000). Modelling the uranium-proton ion exchange in biosorption. Environ. Sci. Technol., 33, 4049-4058.
- YU Q.M., J.T. MATHEICKAL, P.H. YIN, P. KAEWSARN (1999). Heavy metal uptake capacities of common marine macro algal biomass. Water Res., 33, 1534-1537.
- ZHOU J.L. and R.J. KIFF (1991). The uptake of copper from aqueous solution by immobilised fungal biomass. J. Chem. Technol. Biotechnol., 52, 317-330.
- ZOUMIS T., W. CALMANO, U. FORSTNER (2000). Demobilization of heavy metals from mine waters. Acta Hydrochim. Hydrobiol., 28, 212-218.
Liste des figures
Figure 1
Main segments of alginic acid: A) a poly(D-mannuronosyl segment and B) a poly(L-guluronosyl) segment.
Principales parties de l’acide alginique : A) une portion poly(D-mannuronosyl, et B) une portion poly(L‑guluronosyl).
A
B
Figure 2
The chemical structure of lignin.
Structure chimique de la lignine.
Figure 3
The chemical structure of chitin.
Structure chimique de la chitine.
Figure 4
The chemical structure of chitosan.
Structure chimique du chitosan.
Liste des tableaux
Table 1
Studies on the metal sorption using seaweeds
Études portant sur l’adsorption des métaux par utilisation d’algues macroscopiques
References |
Seaweeds |
Metals |
Studied parameters |
|---|---|---|---|
Aravindhanet al. (2004) |
Turbinaria sp. |
Cr(III) |
Pre-treated with H2SO4, CaCl2 and MgCl2 |
Axtellet al. (2003) |
Microspora sp. |
Ni(II), Pb(II) |
Batch and semi batch process, kinetic study |
borba et al. (2006) |
Sargassum filipendula |
Ni(II) |
Pre-treatment, fixed bed column, mathematical modeling |
cepriáet al. (2006) |
Ulva rigida |
Au(III), Hg(II), Ag(I), |
Biomass modified electrode, factors affecting biosorption |
Chaisuksant (2003) |
Gracilaria fisheri (red marine algae) |
Cd(II), Cu(II) |
Langmuir model, effect of pH, pre-treated with CaCl2. |
Cossichet al. (2004) |
Sargassum sp. |
Cr(III) |
Fixed-bed column, mass balance |
Da Costa and De França (1996) |
Codium sp., Colpomenia sp., Gelidium sp., Padina sp., Sargassum sp., Ulva sp. |
Cd(II) |
Langmuir and Freundlich models, ion-exchange mechanism, effect of pH |
Da Costaet al. (1996) |
Sargassum sp. |
Al(III), Ca(II), Cd(II), Mg(II), Na(I), Zn(II) |
Synthetic and natural effluents, kinetic |
Gonget al. (2005) |
Spirulina maxima |
Pb(II) |
pH, contact time, biomass concentration, Freundlich model, desorption, pre-treated with CaCl2. |
Guptaet al. (2001) |
Spirogyra sp. |
Cr(VI) |
Batch sorption, kinetic studies, effect of pH, Langmuir model |
Holanet al. (1993) |
Ascophyllum nosodum, Fucus vesiculosus |
Cd(II) |
Cross-linking, desorption |
Holan and Volesky (1994) |
Ascophyllum nosodum, Fucus vesiculosus |
Ni(II), Pb(II) |
Langmuir model, effect of pH, crosslinked with formaldehyde, bis(ethenyl)sulfone and 1-chloro-2,3-epoxypropane |
Kaewsarn and YU (2001) |
Padina sp. |
Cd(II) |
Batch and column experiments, kinetic studies, effect of pH |
Kaewsarnet al. (2001) |
Durvillaea potatorum |
Cu(II) |
Co-ions effect (light and heavy metals, EDTA) |
Kaewsarn (2002) |
Padina sp. |
Cu(II) |
Langmuir isotherm, kinetic studies, effect of pH, batch and fixed-bed experiments |
khani et al. (2006) |
Cystoseira indica |
U(VI) |
Batch sorption, kinetic modelling, effect of pH, protonated algae, Ca-pretreated algae |
Kratochvillet al. (1998) |
Sargassum sp. |
Cr(III), Cr(VI) |
Protonated seaweed, pH optimization |
Kuyucak and Volesky (1988) |
Ascophyllum nodosum, Chondrus crispus, Halimeda opuntia, Palmaria palmata, Porphyra tenera, Sargassum natans |
Ag(I), Au(III), Cd(II), Co(II), Cu(II), Pb(II), U(VI), Zn(II) |
Algal biomass, dead and living yeast, comparison with activated carbon, anion exchange resin (IRA-400) and cations exchange resin (Duolite-C20) |
kumaret al. (2006) |
Ulva fasciata sp. |
Cu(II) |
Effect of pH and algae concentration, effect of particle size, adsorption kinetics, pseuso-first and pseudo-second models, adsorption equilibrium |
Lauet al. (2003) |
Ulva lactuca |
Cu(II), Ni(II), Zn(II) |
Langmuir isotherm, effect of cations and anions, kinetic study, effect of pH, seaweed biomass concentration, effect of reaction time,desorption |
Lee and Volesky (1997) |
Sargassum fluitans |
Al(III), Ca(II), K(I), Mg(II), Na(I) |
Light metals affinity, proton uptake |
Leuschet al. (1995) |
Ascophyllum nodosum Sargassum fluitans |
Cd(II), Cu(II), Ni(II), Pb(II), Zn(II) |
Crosslinked with formaldehyde, glutaraldehyde and embedded in polyethylene imine, Langmuir, Freundlich and Dubinin-Radushkevich models, effect of particle size |
lodeiroet al. (2006) |
Cystoseira baccata |
Cd(II), Pb(II) |
Kinetic experiments, temperature effect, Langmuir-Freundlich model, effect of salinity on metal uptake, FTIR analysis |
luoet al. (2006) |
Laminaria japonica |
Pb(II) |
Chemical modification, Langmuir isotherm, effect of pH, effect of solid/liquid ratio. |
Matheickal et al. (1997) |
Ecklonia radiata |
Cu(II) |
pH effect, effect of EDTA, acetate, nitrate and chloride on metal uptake, packed bed system |
Matheickal and YU (1997) |
Phellinus badius |
Pb(II) |
Langmuir isotherm, kinetic studies, effect of pH, batch and fixed-bed experiments |
naja and Volesky (2006) |
Sargassum fluitans |
Cu(II), Zn(II), Cd(II) |
Equilibrium and sorption models |
Oferet al. (2003) |
Padina pavonia, Sargassum vulgaris |
Cd(II), Ni(II) |
Kinetic studies, desorption studies, Langmuir isotherm |
Parket al. (2004) |
Ecklonia sp. |
Cr(III), Cr(VI) |
Redox reaction with the biomass, thermal treated biomass, SEM, BET, FTIR characterization |
Prasheret al. (2004) |
Palmaria palmate (red algae) |
Cd(II), Cu(II), Ni(II), Pb(II), Zn(II) |
Freundlich, Langmuir and Brunauer Emmer and Teller (BET) models, effect of contact time, pH, initial concentration and temperature |
Senthilkumaret al. (2006) |
Gracilaria crassa, Gracilaria edulis, Hypnea valentiae, Ulva lactuca, Ulva reticula, Codium tomentosum, Chaetomorpha antennina, Turbinaria conoides, turbinaria ornata, Sargassum polycystium |
Zn(II) |
Batch experiments, Langmuir, Freundlich, Redlich-Peterson and Sips models, columns experiments, biosorption kinetics, sorption thermodynamics, influence of co-ions, desorption studies |
tsui et al. (2006) |
Sargassum hemiphyllum |
Ag(I), As(V), Cd(II), Co(II), Cd(II), Cr(III), Cr(VI), Mn(II), Ni(II), Pb(II), Zn(II) |
Ca-treated biomass, different ionic strengths, binding mechanism |
Volesky (1994) |
Sargassum natans, Sargassum fluitans, Sargassum vulgaris, Ascophyllum nodosum, Palmaria palmata, Chondrus crispus, Halimeda opuntia, Fucus vesiculosis, Padina gymnospora, Codium taylori |
Cd(II), Pb(II) |
|
Yuet al. (1999) |
Ascophyllum nodosum, Lessonia flavicans, Lessonia nigresenseLaminaria japonica, Laminaria hyperbola, Ecklonia maxima, Ecklonia radiate, Durvillaea potatorum |
Cd(II), Cu(II), Pb(II), U(VI) |
Langmuir isotherm, radionuclides, effect of grown media |
Table 2
Recent studies on the adsorption capacities (mg/g) of marine algae for selected heavy metals.
Études récentes sur la capacité d’adsorption (mg/g) des l’algues marines pour des métaux lourds sélectionnés.
References |
Sorbents |
Cd(II) |
Cu(II) |
Cr(III) |
Ni(II) |
Zn(II) |
|---|---|---|---|---|---|---|
ARAVINDHAN et al. (2004) |
T. ornata |
|
|
31 |
|
|
CHAISUKANT (2003) |
G. fisheri |
71 |
46 |
|
|
|
COSSICH et al. (2004) |
Sargassum sp. |
|
|
68 |
|
|
KUMAR et al. (2006) |
Ulva fasciata sp. |
|
26.9 |
|
|
|
LAU et al.(2003) |
Ulvas sp. 1 Ulva lactuca Ulva sp. 3 |
|
47 55 51 |
|
10 19 16 |
34 44 31 |
LODEIRO et al. (2004) |
S. muticum |
154 |
|
|
|
|
LODEIRO et al. (2006) |
C. baccata |
101 |
|
|
|
|
OFER et al. (2003) |
S. vulgaris P. pavonia |
135 88 |
|
|
62 34 |
|
PAVASANT et al. (2006) |
Caulerpa lentillifera |
1.59 |
5.56 |
|
|
2.66 |
Table 3
Studies on the metal sorption using alginate products.
Études portant sur l’adsorption des métaux sur les produits d’alginate.
References |
Sorbents |
Metals |
Studied parameters |
|---|---|---|---|
Al-Rubet al. (2004) |
Alginate beads |
Ni(II) |
Effect of immobilized algae cells (living and death), effect of pH and initial concentration, alginate beads, Langmuir model, sorption desorption cycle |
Aksuet al. (1998) |
Ca-alginate beads agarose, Chlorella vulgaris |
Cu(II) |
Packed-bed column, flow rates |
Chenet al. (1993) |
Alginate beads |
Cu(II) |
Linear absorption model, diffusion coefficient, density of alginate |
Cristet al. (1994) |
Vaucheria, Rhizoclonium, Ca-alginate powder |
Ag(II), Al(III), Ba(II), Cd(II), Cu(II), La(III), Mg(II), Pb(II), Sr(II) Zn(II) |
Ion exchange constant, rate of removal of sorbed metal, ion exchange model |
Fourest and Volesky (1997) |
Alginate extraction from Sargassum fluitans, Ascophyllum nodosum, Fucus vesiculosus, Laminaria japonica |
Ca(II), Cd(II), Cu(II), Pb(II), Zn(II) |
Characterization of physical properties of alginate by potentiometric titration and 13C NMR, metal binding |
Gotohet al. (2004) |
Alginate gel beads |
Cu(II), Mn(II) |
Doped alginate beads with cyanogens bromide and 1,6-diaminohexane, FTIR and SEM characterization |
Hirai and Odani (1994) |
Alginic acid film, sodium alginate film, alginate-Co complex |
Co(II) |
Absorption, desorption, diffusion coefficient, film characterization |
Huanget al. (1996) |
Alginate and chitosan beads |
Cu(II), Ni(II) |
Metal selectivity, particle size, kinetic, isotherm, effect of pH |
IBÁÑEZ and Umetsu (2002) |
Protonated alginate beads |
Co(II), Cr(III), Cu(II), Ni(II), Zn(II) |
Metal uptake, beads morphology, effect of protonation, effect of: ionic strength, pH and protonation |
IBÁÑEZ and Umetsu (2004) |
Protonated dry alginate beads |
Cr(III) |
Batch tests, effect of pH, mechanism, EPMA-EDX analysis |
Janget al. (1995) |
Alginate beads |
Co(II), Cu(II) |
In-situ crosslinking, metal selectivity, fluidized bed reactor, Langmuir model |
Janget al. (1999) |
Alginate beads |
Cu(II), Zn(II) |
In-situ crosslinking, extended Langmuir model, binding group density, binding stability constant |
Jeonet al. (2002) |
Carboxylated alginic acid |
Pb(II) |
Carboxylated alginic acid using KMnO4, FTIR and 13C NMR characterization, elemental analysis, desorption |
Jeonet al. (2005) |
Carboxylated alginic acid |
Cu(II), Pb(II) |
Effect of ionic strength and organic material effect, desorption |
Karagunduzet al. (2006) |
Dried alginate beads |
Cu(II) |
Kinetic sorption, equilibrium experiment, surfactant entrapped dried alginate beads. |
Lu and Wilkins (1996) |
Sacharomyces cerevisiae immobilized in alginate gels |
Cd(II), Cu(II), Zn(II) |
Caustic treatment, metal desorption, biosorbent reactivation |
Nestle and Kimmich (1996) |
Alginate beads |
Cu(II) |
NMR analyses, spatial distribution, diffusion coefficient |
PAPAGEORGIOUet al. (2006) |
Alginate beads, alginate extracted from Laminaria digitata |
Cu(II), Cd(II), Pb(II) |
Alginate beads characterization, batch metal uptake, Langmuir, Freundlich and Sips models, kinetic model, batch kinetic model |
Park and Chae (2004) |
Alginate beads, alginate capsules, alginate gel coated |
Pb(II) |
Regeneration of alginate adsorbent, SEM characterization |
Seki and Suzuki (1996) |
Alginic acid and humic acid membranes |
Pb(II) |
Equilibrium parameters, complexation model |
Shimizu and Takada (1997) |
Alginate fibers |
Bi(III), Cu(II), Pb(II), Sr(II) |
Effect of nitric acid, fibers characterization |
Singhalet al. (2004) |
Chlorella pyrendoidosa impregnated beads Ca-alginate |
U(IV), U(VI) |
Column, desorption, kinetic, FTIR characterization |
Veglioet al. (2002) |
Ca-alginate beads |
Cu(II) |
Effect of pH, Langmuir isotherm |
Table 4
Recent studies on the adsorption capacities (mg/g) of alginate for selected heavy metals.
Études récentes sur la capacité d’adsorption (mg/g) de l’alginate pour des métaux lourds sélectionnés.
References |
Sorbents |
Cu(II) |
Cr(III) |
Cr(VI) |
Ni(II) |
Pb(II) |
|---|---|---|---|---|---|---|
AL-RUB et al. (2004) |
Alginate beads Free dead algal cell Immobilized dead algal cells |
|
|
|
26 14 31 |
|
BAJPAI et al. (2004) |
Bio-polymeric (Ca and gelatin) |
|
|
0.83 |
|
|
IBÁÑEZ and UMETSU (2004) |
Protonated dry alginate beads |
|
112 |
|
|
|
Ngomsiket al. (2006) |
Magnetic alginate microcapsule |
|
|
|
26 |
|
OZDEMIR et al. (2005) |
Alginate beads Alginate-Extracellular polysaccharide |
96 126 |
|
|
59 71 |
|
PARK and CHAE (2004) |
Alginate beads Alginate capsule |
|
|
|
|
450 1540 |
Table 5
Treatment options for the removal of heavy metals (adapted from ADERHOLD et al., 1996).
Options de traitement pour l’enlèvement des métaux (adapté d’ADERHOLD et al., 1996).
Treatment options |
Advantages |
Disadvantages |
|---|---|---|
Lime precipitation |
Relatively inexpensive Bulk removal |
Non selective |
Ion exchange |
Multiple ion-exchange resins High specificity for heavy metal Several sorption and desorption cycles No sludge production Electrolysis allows metal recycling |
High capital and operating costs Economic of these process depends on energy price and the amount of electricity used per treated volume of solution |
|
Membrane processes Osmosis Reverse osmosis |
|
Very specialized application Limited flow rate Membrane instability in salt and acid conditions Prohibitive cost |
Adsorption processes |
Versatile, simple Selectively sorbed the sorbate Low cost technology |
|
Table 6
Studies on the adsorption of different metals using natural adsorbents.
Études portant sur l’adsorption de différents métaux sur des adsorbants naturels.
Metals |
References |
|---|---|
Aluminium (Al) (III) |
Crist et al. (1994), Orhan and Büyükgüngör (1993), CUI et al. (2006) |
Antimony (Sb) (III) |
Coupal and Lalancette (1976), Masri and Friedman (1974) |
Arsenic (As) (II, V) |
Loukidouet al. (2003), Masri and Friedman (1974) |
Barium (Ba) (II) |
Cristet al. (1994), Smithet al. (1977) |
Bismuth (Bi) (III) |
Masri and Friedman (1974), Shimizu and Takada (1997) |
Cadmium (Cd) (II) |
Volesky and Prasetyo (1994), YU et al. (1999) |
Calcium (Ca) (II) |
Fiset et al. (2002), Fourest and Volesky (1997) |
Cerium (Ce) (III) |
Masri and Friedman (1974) |
Chromium (Cr)(III, VI) |
Baileyet al. (1992), FISHER et al. (1984) |
Cobalt (Co) (II) |
Flynnet al. (1980), Kuyucak and Volesky (1988) |
Copper (Cu) (I, II) |
MCKAY et al. (1999), YU et al. (1999), CUI et al. (2006) |
Europium (Eu) (III) |
Andreset al. (1993) |
Gold (Au) (III) |
Kuyucak and Volesky (1988), Nakajima (2003) |
Iridium (Ir) (IV) |
Ruizet al. (2003) |
Iron (Fe) (II, III) |
Fisetet al. (2002), Nassaret al. (2004), CUI et al. (2006) |
Lanthanum (La) (III) |
Bloom and McBride (1979), Cristet al. (1994) |
Lead (Pb) (II) |
Holan and Volesky (1994),YU et al.(1999), Murathan and Bütün (2006) |
Magnesium (Mg) (II) |
Crist et al. (1994), Fisetet al. (2002), CUI et al. (2006) |
Manganese (Mn) (II) |
Fiset et al. (2002), Nassaret al. (2004), CUI et al. (2006) |
Mercury (Hg) (I, II) |
FISHER et al. (1984), Viraraghavan and Kapoor (1994) |
Molybdenum (Mo) (VI) |
Guibal et al. (1999), Sakagushi et al. (1981) |
Nickel (Ni) (II) |
Flynnet al. (1980), Leuschet al. (1995) |
Osmium (Os) (IV) |
Ruizet al. (2003) |
Palladium (Pd) (II) |
Baba and Hirakawa (1992), Guibal et al. (2001) |
Platinum (Pt) (IV) |
Baba and Hirakawa (1992), Guibal et al. (2001) |
Radium (Ra) (II) |
TSEZOS (1997), TSEZOS and KELLER (1983) |
Silver (Ag) (I) |
FISHER et al. (1984), Flynnet al. (1980) |
Sodium (Na) (I) |
Fisetet al. (2002), Spintiet al. (1995) |
Strontium (Sr) (II) |
Shimizu and Takada (1997); Small et al. (1999) |
Technetium (Tc) (VII) |
Garnhamet al. (1992a, 1993b) |
Thallium (Tl) (I) |
Masri and Friedman (1974) |
Thorium (Th) (IV) |
Masri and Friedman (1974), Tsezos and Volesky (1981) |
Titanium (Ti) (IV) |
Parkash and Brown (1976) |
Uranium (U) (IV, VI) |
Guibal et al. (1994), Tsezos and Volesky (1982) |
Vanadium (V) (V) |
Guibal et al. (1994) |
Ytterbium (Yb) (III) |
Andres et al. (1993) |
Zinc (Zn) (II) |
Artola and Rigola (1992); Kuyucak and Volesky (1988) |
Zirconium (Zr) (IV) |
Garnhamet al. (1993a), Parkash and Brown (1976) |
Table 7
Some examples of the microorganisms studied for the metal recovery from solutions.
Exemples de microorganismes étudiés pour la récupération de métaux en solution.
Microorganisms |
References |
|---|---|
Bacteria |
|
Bacillussubtilis and spp. |
Beveridgeet al. (1982), Cotoraset al. (1992), GREEN-RUIZ (2006) |
Micrococcus spp. |
Cotoraset al. (1992), Loet al. (2003) |
Mycobacterium spp. |
Andres et al. (1993) |
Pseudomonas spp. |
Cabral (1992), Lopezet al. (2002), D’SOUZA et al. (2006) |
Streptomyces spp. |
Friss and Myers-Keith (1986), Mattuschka and Straube (1993) |
Zooglea ramigera |
Aksu et al. (1992), Norberg and Persson (1984) |
Yeasts |
|
Candida spp. |
Aksu and Donmez (2001) |
Candida utilis |
kujan et al. (2006) |
Saccharomyces cerevisiae |
Kuyucak and Volesky (1988), Volesky et al. (1993) |
Fungi |
|
Aspergillus niger |
Venkobachar (1990) |
Aureobasidium pullulans |
Gadd and De Rome (1988) |
Cladosporium resinae |
Gadd and De Rome (1988) |
Funalia trogii |
ARICAet al. (2004) |
Ganoderma lucidum |
Venkobachar (1990) |
Penicillium spp. |
Loukidouet al. (2003), Sayet al. (2003) |
Rhizopus arrhizus |
Fourest and Roux (1992), Tsezos and Volesky (1982) |
Trametes versicolor |
Bayramogluet al. (2003) |
Micro-algae |
|
Chlorella vulgaris and spp. |
Aksu and Acikel (1999), Mehtaet al. (2002) |
Chlamydomonas spp. |
Garnham et al. (1992a), Sakaguchiet al. (1981) |
Eudorina spp. |
Tien (2002) |
Euglena spp. |
Mannet al. (1988) |
Scenedesmus spp. |
Garnhamet al. (1993a), Sakaguchiet al. (1981) |
Synechocystis aquatilis |
ergene et al. (2006) |
Cyanobacteria |
|
Anabaena spp. |
Garnhamet al. (1993b), Tien (2002) |
Nostoc spp. |
Fernandez-Pinaset al. (1991), Hassett et al. (1981) |
Oscillatoria spp. |
Fisheret al. (1984), Tien (2002) |
Synechoccus spp. |
Garnham et al. (1993a, b), Sakaguchiet al. (1978) |
Other |
|
Activated sludge |
Artola and Rigola (1992), Hammainiet al. (2003) |
Table 8
Agricultural wastes studied for the metal recovery from solutions.
Déchets agricoles étudiés pour la récupération de métaux en solution.
Wastes |
References |
|---|---|
Banana pith and peels |
Annaduraiet al. (2003), Lowet al. (1995) |
Canola meal |
Al-Asheh and Duvnjak (1996) |
Carrot residues |
Nasernejadet al. (2005) |
Cassava fibre |
ABIAet al. (2006) |
Chicken feathers |
Al-Ashehet al. (2002) |
Cocoa shells |
Fiset et al. (2002), Meunieret al. (2003a, b, 2004) |
Coconut byproducts |
Randallet al. (1974), OFOMAJA and HO (2007), Mohan et al. (2006) |
Coffee residues |
Minamisawaet al. (2002) |
Corn cobs and roots |
Bosincoet al. (1996), Goldberg and Grieve (2003) |
Grape stalks |
Fiol et al. (2006), Escuderoet al. (2006) |
Indian mustard |
Crist et al. (2004) |
Modified wool |
Marshall and Champagne (1995) |
Nut and walnut shells |
Orhan and Büyükgüngör (1993) |
Olive mill residues |
Gharaibehet al. (1998), Veglioet al. (2003) |
Onion peels |
Kumar and Dara (1982) |
Orange peels |
Ajmalet al. (2000), Masriet al. (1974) |
Palm kernel fibre |
Ho and OFOMAJA (2006) |
Peanut skins |
Chamarthyet al. (2001), Randall et al. (1974) |
Petiolar felt-sheath of palm |
Iqbal and Saeed (2002) |
Rice byproducts |
Ajmal et al. (2003), Montanheret al. (2005) |
Sheep manure wastes |
Al-Rubet al. (2003) |
Sunflower seed peel |
Ozdemiret al. (2004) |
Tea leaves |
Orhan and Büyükgüngör (1993), Tee and Khan (1988) |