Résumés
Abstract
The occurrence of the mesophilic motile Gram negative non enterobacterial species A. hydrophila in the wild is a major problem that deserves to be resolved since it is a potentially pathogen able to enter into a non-culturable state on routine bacteriological plating media. These non-culturable forms can be detected by several direct or indirect visualization methods. This species has frequently been isolated from pathological forms in fish farming marine areas, especially near wastewater discharges. Consequently, we studied A. hydrophila in marine water microcosms placed during a 24 hour period in treated waste waters and compared with the homologous strain not placed in the same conditions. Thus, two kinds of microcosms were prepared using filtered and autoclaved marine water or waste water, inoculated by A. hydrophila and maintained at 25°C in darkness. The results obtained indicated that A. hydrophila population incubated at 25°C in marine water declined rapidly (3.21 log units in plate count number) during the first three days. Additionally, we noted that A. hydrophila incubated in marine water after a previous treatment in waste water declined progressively to 2.74 log units (in plate count number). Nevertheless, we showed no significant variations of the number of total bacterial cells for A. hydrophila developed in marine water after prior treatment in waste water, despite the appearance of the VBNC form. During this state, rods of normal size, elongated cells and cocci were obtained. Concomitantly, we determined several changes in biochemical and antimicrobial patterns of stressed A. hydrophila, notably the acquisition of adipate metabolization and an increase of resistance to antimicrobial compounds, especially for A. hydrophila incubated in marine water after treatment in waste water.
Key words:
- Aeromonas hydrophila,
- wastewater,
- marine water,
- survival,
- VBNC form
Résumé
A. hydrophila (mésophile, Gram négative, mobile) compte parmi les espèces non entérobactériennes opportunistes du milieu aquatique. Son isolement ou son dénombrement en milieu marin selon les méthodes classiques est compromis par l’acquisition de la forme viable non cultivable (VNC) chez la bactérie dont la visualisation repose sur des méthodes moins usuelles, notamment la cytométrie en image ou en flux. En pathologie aquacole, A. hydrophila compte parmi les espèces les plus fréquemment isolées, notamment dans les zones de rejets. Le présent travail a été effectué en vue d’étudier la survie d’A. hydrophila en eau de mer après transit en eaux usées domestique. Ainsi, deux types de microcosmes ont été utilisés, le premier rempli d’eau de mer filtrée, stérile et placé à 25 °C à l’obscurité; le second est rempli d’eau usée filtrée, stérile et placée pendant 24 h à l’obscurité avant transfert en eau de mer. Les caractéristiques intrinséques : cultivabilité, forme cellulaire, profils biochimiques et antibiotypiques ont été suivis au cours de ce stress. Les résultats obtenus indiquent que la cultivabilité d’A. hydrophila placée directement dans l’eau de mer diminue rapidement durant les trois premiers jours d’incubation (3,21 Ulog). Aussi, nous avons noté une réduction progressive de la cultivabilité d’A. hydrophila placée en eau de mer après transit en eaux usées (2,74 Ulog). Néanmoins, le nombre de cellules totales ne montre pas de variation significative tout le long de la période de suivi (30 jours), avec l’apparition de la forme Viable Non Cultivable (VNC). Au cours de ce stress, nous avons observé des cellules de formes bacillaires, allongées et arrondies réduites. Parallèlement, nous avons trouvé qu’A. hydrophila ayant séjourné en eau de mer (avec ou sans passage en eaux usées) a subi différentes modifications ayant porté aux caractéristiques biochimiques et antibiotypiques, dont les plus remarquables sont la capacité à métaboliser l’adipate et l’acquisition de nouvelles résistances aux antibiotiques, notamment après passage en eaux usées.
Mots clés:
- Aeromonas hydrophila,
- eaux usées,
- eau salée,
- survie,
- forme VNC
Corps de l’article
1. Introduction
Aeromonas hydrophila is an opportunistic gram negative non‑enterobacterial species with wide distribution in aquatic habitats (Krovaceket al., 1994; Kersteret al., 1995; Wiklund, 1995 and Maalejet al., 2004). The ubiquitous nature of Aeromonas species in aquatic environments is dangerous, especially for fish and shellfish species as it was the causative agent of several diseases in aquaculture (Shariff, 1998 and Santoset al., 1999). Several authors noted that the survival of A. hydrophila in aquatic environment was inhibited by entrance into a viable but non‑cultivatable (VBNC) state, due to stress induced by aquatic conditions for the bacterial cell, which remained metabolically active, but unable to undergo the sustained cellular division required for growth in different conditions (Duncanet al., 1994; Maalejet al., 2004; Monfortet al., 1999 and Oliveret al., 1991). In Tunisian coastal ecosystems, this species was the most frequent isolated from different categories of marine samples (water, sediment, fish, mussels, and clams) (Bouamama, 2001; Dallali, 2001 and El Bouret al., 2001). That A. hydrophila might enter into a VBNC state, in which it would be undetectable by routine methods, presents an important concern, especially as it remains virulent. The purpose of the present study was to assess the effect of treated waste water on the behaviour of A. hydrophila incubated in marine water and the probable changes on culture behaviour, cellular aspects, and biochemical and antibiotic characteristics.
2. Material and methods
2.1 Bacterial strain, biochemical and antibacterial characteristics
A. hydrophila used throughout the study was previously isolated from the eel Monopterus albus in the department of Veterinary Microbiology of the Agricultural and Veterinary University of Denmark, and then stored in the Laboratory of Pathology of the National Institute of Sea Sciences and Technology (INSTM) located in Salammbo, 15 km north of Tunis (Tunisia). The strain was streaked on Muller Hinton agar plates (BIORAD, France) and incubated at 32°C for 24 h before use. The biochemical features described by the sending laboratory were confirmed using conventional tests (Gram staining, motility, oxidase and catalase) and strips Api 20 NE systems (Biomerieux, France) (Borrelet al., 1998; Goni-Urrizaet al., 1999). The whole profile responses of A. hydrophila are shown in Table 1.
Table 1
Biochemical characteristics of Aeromonas hydrophila (B3).
Charactéristiques biochimiques d’Aeromonas hydrophila (B3).
|
Gram |
Mobilité |
Hémolyse |
Ox |
Cat |
DCA |
DCL |
DCO |
URE |
IND |
VP |
GLU |
O‑ser |
AMX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
B3 |
- |
+ |
+ |
+ |
+ |
+ |
- |
- |
+ |
+ |
+ |
+ |
O13 |
R |
Note: (+) positive reaction, (-) negative reaction, Ox: oxidase, Cat : catalase, DCA: arginine decarboxylase, DCL: lysine decarboxylase, DCO: ornithine decarboxylase, URE: urease, IND: indole, VP: Voges-Proskauer, Glu: glucose, O-ser: O-serotype, AMX: amoxicilin, R: resistant.
We followed for antibacterial assays the antibiogramm method of Chabbert et al. (1982). We used 11 antibiotic disks, as follows: Streptomycin (10 UI), Tobramycin (10 UI), Chloramphenicol, Tetracycline (30 UI), Furans (300 UI), Trimethoprim-sulfamethoxazol (1.25 UI), Rifampicin (30 µg), oxalinic acid (10 µg), Novobiocin (30 µg), Amoxicillin (10 UI) and Oxacillin (10 UI) (BIORAD, France) (Hamzeet al., 1998). Organisms were classified after 24 h incubation at 32°C on Muller Hinton agar plates (BIORAD, France) as sensitive, intermediate or resistant following French National Guidelines (S.F.M, 1998).
2.2 Inoculum preparation
A bacterial suspension was obtained by growing the strain of A. hydrophila in Trypto Casein Soja Broth (TSB, BIORAD) at 32°C for 18 h (stationary growth phase) and harvesting by centrifugation (6000 x g, 10 min). The pellet was washed three times and re-suspended in sterile saline solution (NaCl 9‰) in order to obtain the initial cellular inoculum.
2.3 Preparation of microcosms
Two Pyrex bottles (500 mL) were used as microcosms. Each one contained 100 mL of natural marine water (salinity: 30.5‰, dissolved O2: 7.65, pH: 8.65) filtered through 0.2 µm pore size membranes (Nuclepore). Bacterial cells were suspended in duplicate microcosms at a concentration of 106 colony forming units per mL (CFU/mL). Microcosms were stored at room temperature (25°C) in darkness for 30 d.
Another Pyrex bottle (100 mL) filled with domestic wastewater that had been treated in an aerated lagoon (Kaalat Andalous, 15000 inhabitants), filtered through 0.45 µm and 0.2 µm pore size membranes (Nuclepore) and then sterilized (121°C, 20 min). We then inoculated with the bacterial suspension and stored the bottles at room temperature (25°C) in darkness for 24 h (Dupray and Derrien, 1994). Bacterial cells were harvested by filtration of the whole suspension through 0.2 µm cellulose nitrate filters (Sartorius) and the filters were inoculated into the marine water microcosms for 30 d. The experiment was conducted once, in duplicate (two bottles per sample).
Samples from each microcosm were taken in duplicate immediately after inoculation and after 1, 3, 4, 7, 15 and 30 d. These samples were used for cell staining and enumeration.
2.4 Colony and cell counting
One mL of each sample was serially diluted in sterile saline solution (9‰ NaCl) for subsequent cell counting. Plate counts were performed by plating 0.1 mL of each sample in duplicate on Muller Hinton agar and incubating the plates at 32°C for 24 h prior to CFU determination.
2.5 Cells staining analysis
One mL of the sample was removed and diluted in sterile saline solution (9‰ NaCl). The suspension was stained with SYBR Green I (SGI) (Molecular Probes). SGI was used because it has a high affinity for DNA. This latter was stained by adding 5 µL of SGI and incubated for 15 min. at room temperature (Lebaronet al., 1998). The stained cells were filtered through polycarbonate black membranes (pore size 0.2 µm, diameter 25 mm, Millipore). The filter was placed on a glass slide and a drop of immersion oil was placed on top followed by a cover slip which was mounted on top of each filter. The concentration of total cells was determined with a POLYVAR epifluorescence microscope by examination of the slides under immersion oil with a 1000x objective lens. Counting was carried out after excitation at 490 nm and examination of 30 microscopic fields per slide. Results are given for each experiment as the number of bacteria per mL of the original sample (Lebaronet al., 1994; Troussellieret al., 1985).
2.6 Morphological and biochemical modifications:
Modifications to the morphological features were determined after the full time incubation in the microcosms (30 d). The strains were then streaked onto Muller Hinton agar plates and incubated under the same conditions as the initial study (18 to 24 h at 30-32°C). Colonies were characterized by their shape, size, surface texture, colour and opacity. Gram staining was carried out to determine eventual cellular shape modifications. For the biochemical characterization, all the enzymatic and metabolic tests were carried out as described previously (Borrelet al., 1998; Goni-Urrizaet al., 1999).
2.7 Antibacterial sensitivity modifications:
To detect eventual changes to antibiotic sensitivity, we used an agar diffusion method described previously (Chabbert, 1982).
3. Results and Discussion
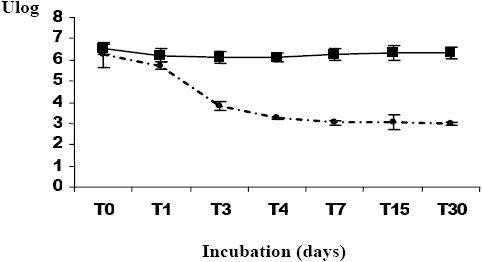
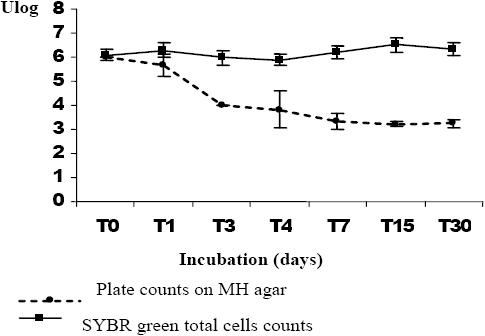
The results of the survival of A. hydrophila incubated in filtered seawater for thirty days showed a rapid decline in the plate count number during the first three days (3.21 log units) (Figure 1a). A similar decline was observed during the remaining time of the experiment. According to Laaberki (1999), Lakhal (2003) and Maalejet al. (2004), the decline after transfer into the seawater microcosms is already observed during the first days of incubation (from Day 2 to Day 7). During similar experiments, we observed that the total cell number revealed by the staining method did not change throughout the survey. VBNC forms appeared during the first days of the microcosm incubation (Figure 1a). Similar results were reported by Laaberki (1999), Lakhal (2003) and Maalejet al. (2004) for the survival of A. hydrophila in marine water, and for Brandiet al. (1999), Maryet al. (2002) and Messiet al. (2002) for survival ability of A. hydrophila in different water types. The results obtained for A. hydrophila survival in marine water after incubation in waste water showed a greater progressive decline in the plate count number (2.74 log units) (Figure 1b). The total cell number did not vary significantly. These results show that the change between total cells number and counting plate number was less important in the case of bacteria that had been treated in waste water prior to being transferred into marine water (2.83 log units) than for species incubated directly in marine water (3.5 log units). Dupray and Derrien (1994) noted that the pre-adaptation acquired by the bacteria was due to the high loading of the organic matter present in wastewater ponds. Additionally, we observed a reduction in size of A. hydrophila colonies after incubation in the microcosms, despite the appearance of ovoid and elongated cells for bacterial strains for the two kinds of microcosms (Figure 2). According to Huisman et al. (1996), the decrease in size may be due to condensation of the cytoplasm and reduction of the periplasm volume. In Tunisia, Lakhal (2003) described the morphological modifications observed for A. hydrophila incubated in several categories of marine water. Maryet al. (2002) noted the presence of elongated cells of A. hydrophila incubated under starvation conditions.
Figure 1
Survival rates of Aeromonas hydrophila incubated in filtered seawater (30 d). (a) Aeromonas hydrophila incubated directly in seawater (30 d); (b) Aeromonas hydrophila incubated in seawater after a previous incubation in wastewater (24h).
Taux de survie d’Aeromonas hydrophila lors d’incubations dans de l’eau de mer filtrée (30 j). (a) Aeromonas hydrophila incubée directement dans l’eau de mer; (b) Aeromonas hydrophila incubée dans l’eau de mer après un prétraitement dans des eaux usées.
(a)
(b)
Figure 2
The morphological modifications of Aeromonas hydrophila that occurred during incubation in seawater (30 d). (a) Gram stain of A. hydrophila incubated on TSB; (b) Gram stain of A. hydrophila after incubation in seawater with or without transit in wastewater.
Les modifications morphométriques qui se sont produites chez Aaeromonas hydrophila lors d’incubations dans l’eau de mer (30 j). (a) Coloration Gram d’A. hydrophila incubée sur TSB; (b) Coloration Gram d’A. hydrophila après incubation dans l’eau de mer, avec ou sans prétraitement avec des eaux usées.
(a)
(b)
At first A. hydrophila as sub-cultured on TSB (S0) was biochemically characterized by production of oxidase, catalase, arginine dihydrolase, nitrate reductase, urease, esculine reductase, tryptophane desaminase, gelatinase and β-galactosidase and it metabolized almost all the tested carbohydrates (Table 2). A. hydrophila maintained for 30 d in marine water microcosms (S1) acquired the ability to metabolize all the carbohydrates and continued to produce several enzymes (Table 2). In contrast, A. hydrophila incubated in waste water prior to incubation in marine water microcosms (S2) failed to produce numerous enzymes (Table 2). Lakhal (2003) reported similar patterns in A. hydrophila incubated during a long period in different marine water microcosms. Bakhroufet al. (1992) reported a loss of several biochemical characteristics for Salmonella paratyphi B incubated in marine water microcosms.
Table 2
Biochemical characters of Aeromonas hydrophila under different stress conditions in comparison to standard conditions.
Caractéristiques biochimiques d’Aeromonas hydrophila en différentes conditions de stress en comparaison aux conditions standard.
|
NO3 |
TRP |
GLU |
ADH |
URE |
ESC |
GEL |
PNG |
GLU |
ARA |
MNE |
MAN |
NAG |
MAL |
GNT |
CAP |
ADI |
MLT |
CIT |
PAC |
OX |
CAT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Species |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S0 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
- |
- |
+ |
+ |
+ |
+ |
+ |
S1 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
S2 |
+ |
- |
- |
- |
- |
+ |
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
- |
+ |
+ |
+ |
- |
+ |
+ |
Note : (+) positive response; (-) : negative response. NO3 : nitrate reductase; TRP: tryptophane desaminase; GLU: glucose acidification; ADH: arginine dihydrolase; URE: urease; ESC: esculine hydrolyases; GEL: gelatinase; PNPG: p-nitrophenyl-ß-galactopyranoside; Glu: glucose assimilation; ARA: arabinose; MNE: mannose; MAN: mannitol; NAG: N-acetyl-glucosamine; MAL: maltose; GNT: gluconate; CAP: caprate ; ADI: adipate; MLT: malate; CIT: citrate; PAC: phenyl-acetate; OX: oxidase; CAT: catalase.
Antimicrobial susceptibility profiles for standard conditions showed that this strain of A. hydrophila is resistant to six different antibiotics: amoxicillin, oxacillin, furans, rifampicin, streptomycin and novobiocin (Table 3). Similar patterns were reported by Hamze et al. (1998), Bouamama (2001), Dallali (2001), El Bouret al. (2001), Vilaet al. (2002) and Lakhal (2003). A. hydrophila incubated in marine water for 30 days became susceptible to two antibiotics, rifampicin and streptomycin (Table 3). Its increasing susceptibility may be due to alteration of the bacterial membrane as induced by the hyper osmotic conditions (Gutierrez et al., 1995). For the same strain incubated in marine water (during 30 days), after a pretreatment of 24 h in waste water, we noted that A. hydrophila lost susceptibility to three other antibiotics: chloramphenicol, oxalinic acid and trimethoprim-sulfamethoxazole. This strain became resistant to nine different antibiotics. This new resistance may be due to bacterial plasmid or transposon exchange in wastewater (Goni-Urrizaet al., 2000).
Table 3
Modifications of antibiotic profiles of the Aeromonas hydrophila strains after stress conditions.
Modifications des profils antibiotypiques de la souche d’Aeromonas hydrophila après le séjour en conditions de stress.
Strains |
Decreasual susceptibility |
Antibiotics* |
|---|---|---|
S0 |
6 |
Amx –Ox-S-FM -RA-Nov |
S1 |
4 |
Amx –Ox-FM-Nov |
S2 |
9 |
Amx –Ox-S-FM-RA-Nov-C-SXT-AR |
(*) Different antibiotics: AMX: amoxicillin; OX: oxacillin; S: streptomycin; FM: furans; RA: rifampicin; Nov: novobiocin; C: chloramphenicol; SXT: trimethoprim-sulfamethoxazole ; AR: oxalinic acid.
4. Conclusion
A. hydrophila acquired one or several genetic supports conferring resistance to antibiotics during transfer from waste water to marine water. Additionally, the appearance of the VBNC state was more evident for A. hydrophila incubated in waste water prior to being transferred into marine water conditions. This stress induced several morphological, biochemical and antibiotic changes.
Parties annexes
Acknowledgments
The authors are grateful to Dr. Michael Engelbrecht Nielson for providing the strain A. hydrophila B3 used throughout the study.
Bibliographic References
- BAKHROUF A. and M. JEDDI (1992). Modification des caractères culturaux et biochimiques du Salmonella paratyphi B après incubation dans l’eau de mer. Can. J. Microbiol., 38, 871-874.
- BORRELL N., M.J. FIGUERAS and J. GUARRO (1998). Phenotypic identification of Aeromonas genomospecies from clinical and environmental sources. Can. J. Microbiol., 44, 103-108.
- BOUAMAMA K. (2001). Mytilus galloprovincialis de la lagune de Bizerte : populations bactériennes et biomarqueurs non spécifiques. Diplôme des études approfondies DEA. Faculté des Sciences de Tunis, Institut des Sciences et des Technologies de la Mer, 92 p.
- BRANDI G., M. SISTI, F. GIARDINI, G.F. SCHIAVANO and A. ALBONO (1999). Survival ability of cytotoxic strains of motile Aeromonas spp. in different types of water. Lett. Appl. Microbiol., 29, 211-215.
- CHABBERT, Y.A. (1982). L’antibiogramme. In: Bactériologie médicale L. Le Minor, M. Véron, Flammarion (editor). Médecine Science Paris, pp. 205-212.
- COMITÉ DE L’ANTIBIOGRAMME DE LA SOCIÉTÉ FRANÇAISE DE MICROBIOLOGIE (1998). Bull. Soc. Fr. Microbiol., 13, 243-251.
- DALLALI M. (2001). Utilisation d’indicateurs microbiologiques chez Ruditapes decussates et Mytillus galloprovinciallis dans la bio surveillance de la lagune de Bizerte : validation de certains biomarqueurs. Thèse de Doctorat, Faculté des Sciences de Bizerte, pp. 116-117.
- DUNCAN S, L.A. GLOVER, K. KILLHAM and J. PROSSER (1994). Luminescence-based detection of activity of starved and viable but nonculturable bacteria. Appl. Environ. Microbiol., 60, 1308-1316.
- DUPRAY E. and A. DERRIEN (1994). Influence du passage de Salmonella spp. et Escherichia coli en eaux usées sur leur survie ultérieure en eau de mer. Water Res., 29, 1005-1011.
- EL BOUR M., H. ATTIA EL HILLI, R. MRAOUNA and W. AYARI (2001). Bacterial study of mesophillic Aeromonads distribution in shellfish. In: Proceedings of the Fifth international conference on the Mediterranean coastal environment, MEDCOAST, 1, 557-565.
- GONI-URRIZA M., M. CAPDEPUY, N. RAYMOND, C. QUENTIN and P. CAUMETTE (1999). Impact of an urban effluent on the bacterial community structure in the Arga river (Spain), with special reference to culturable Gram-negative rods. Can. J. Microbiol., 45, 826-832.
- GONI-URRIZA M., M. CAPDEPUY, C. ARPIN, N. RAYMOND, P. CAUMETTE and C. QUENTIN (2000). Impact of an urban effluent on antibiotic resistance of riverine Enterobacteriaceae and Aeromonas. Appl. Environ. Microbiol., 66, 125-132.
- GUTIERREZ C., T. ABEE and I.R. BOOTH (1995). Physiology of the osmotic stress response in microorganisms. Int. J. Food. Microbiol., 28, 233-244.
- HAMZE M., F. DABBOUSSI and D. IZARD (1998). Sensibilité à 23 antibiotiques de 83 souches de Aeromonas hydrophila isolées d’eaux libanaises. Cah. Ass. Sci. Eur. Eau Santé, 3, 91-96.
- HUISMAN G.W., D.A. SIEGELE, M.M. ZAMBRANO and R. KOLTER (1996). In: Escherichia coli and Salmonella typhimurium. Cellular and Molecular Biology. Washington, DC, American Society for Microbiology, pp. 1672-1682.
- KERSTERS I., L. VAN VOOREN, G. HUYS, P. JANSSEN, K. KERSTERS and W. VERSTRAETE (1995). Influence of temperature and process technology on the occurrence of Aeromonas species and hygienic indicator organisms in drinking water production plants. Microb. Ecol., 30, 203‑218.
- KROVACEK K, V. PASQUALE, S.B. BALODA, V. SOPRANO, M. CONTE and S. DUMONTET (1994). Comparison of putative virulence in Aeromonas hydrophila strains isolated from the marine environment and human diarrhoeal cases in southern Italy. Appl. Environ. Microbiol., 60, 1379-1382.
- LAABERKI M.H. (1999). Suivi d’Aeromonas isolés des milieux aquatiques et hospitaliers sous l’action des facteurs expérimentaux et naturels. Rapport de stage effectué à l’UMR 5556 CNRS. Université Montpellier II cc93. Laboratoire d’écologie bactérienne des eaux, Montpellier, France, 43 p.
- LAKHAL F. (2003). Étude et suivi de la croissance d’Aeromonas hydrophila en eau de mer. Diplôme des études approfondies DEA. Faculté des Sciences de Tunis, Institut National des Sciences et des Technologies de la Mer, 95 p.
- LEBARON P, M. TROUSSELIER and P. GOT (1994). Accuracy of epifluorescence microscopy count for direct estimates of bacteria numbers. J. Microbiol. Meth., 19, 89‑94.
- LEBARON P., P. CATALA and N. PARTHUISOT (1998): Effectiveness of SYTOX green stain for bacterial viability assessment. Appl. Environ. Microbiol., 64, 2697-2700.
- MAALEJ S., M. DENIS and S. DUNKAN (2004). Temperature and growth-phase effects on Aeromonas hydrophila survival in natural seawater microcosms: role of protein synthesis and nucleic acid content on viable but temporally nonculturable response. Microbiol., 150, 181‑187.
- MARY P., N.E. CHIHIB, O. CHARAFEDDINE, C. DEFIVES and J.P. HORNEZ (2002). Starvation survival and viable but non culturable states in Aeromonas hydrophila. Microbiol. Ecol., 43, 250-258.
- MESSI P., E. GUERRIERI and M. BONDI (2002). Survival of an Aeromonas hydrophila in an artificial mineral water microcosm. Water Res., 36, 3410-3415.
- MONFORT P. and B. BALEUX (1999). Bactéries viables non cultivables : Réalité et conséquences. Bull. Soc. Fr. Microbiol., 14, 201-207.
- OLIVER J.D., L. NILSON and S. KJELLEBERG (1991). Formation of non-culturable Vibrio vulnificus cells and its relationship to the starvation state. Appl. Environ. Microbiol., 57, 2640-2644.
- SANTOS J. A., C.J. GONZALEZ, A. OTERO and M. GARCIA-LOPEZ (1999). Hemolytic activity and siderophore production in different Aeromonas species isolated from fish. Appl. Environ. Microbiol., 65, 5612‑5617.
- SHARIFF, M. (1998). Impact of diseases on aquaculture in the Asia-Pacific region as exemplified epizootic ulcerative syndrome (EUS). J. Appl Ichtyol., 14, 139-144.
- TROUSSELIER M., M. ALBAT, P. ANDRE and B. BALEUX (1985). Dénombrement direct des bactéries dans les milieux aquatiques par microscopie en épifluorescence : distribution et précision des mesures. Rev. Fran. Sci. Eau, 4, 35-49.
- VILA J., F. MARCO, L. SOLER, M. CHACON and M.J. FIGUERAS (2002). In vitro antimicrobial susceptibility of clinical isolates of Aeromonas caviae, Aeromonas hydrophila and Aeromonas veronii biotype sobria. J. Antimicrob. Chemother., 49, 701-102.
- WIKLUND T. (1995). Survival of ‘atypical’ Aeromonas salmonicida in water and sediment microcosms of different salinities and temperatures. Dis. Aquat. Org., 21, 137‑143.
Liste des figures
Figure 1
Survival rates of Aeromonas hydrophila incubated in filtered seawater (30 d). (a) Aeromonas hydrophila incubated directly in seawater (30 d); (b) Aeromonas hydrophila incubated in seawater after a previous incubation in wastewater (24h).
Taux de survie d’Aeromonas hydrophila lors d’incubations dans de l’eau de mer filtrée (30 j). (a) Aeromonas hydrophila incubée directement dans l’eau de mer; (b) Aeromonas hydrophila incubée dans l’eau de mer après un prétraitement dans des eaux usées.
(a)
(b)
Figure 2
The morphological modifications of Aeromonas hydrophila that occurred during incubation in seawater (30 d). (a) Gram stain of A. hydrophila incubated on TSB; (b) Gram stain of A. hydrophila after incubation in seawater with or without transit in wastewater.
Les modifications morphométriques qui se sont produites chez Aaeromonas hydrophila lors d’incubations dans l’eau de mer (30 j). (a) Coloration Gram d’A. hydrophila incubée sur TSB; (b) Coloration Gram d’A. hydrophila après incubation dans l’eau de mer, avec ou sans prétraitement avec des eaux usées.
(a)
(b)
Liste des tableaux
Table 1
Biochemical characteristics of Aeromonas hydrophila (B3).
Charactéristiques biochimiques d’Aeromonas hydrophila (B3).
|
Gram |
Mobilité |
Hémolyse |
Ox |
Cat |
DCA |
DCL |
DCO |
URE |
IND |
VP |
GLU |
O‑ser |
AMX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
B3 |
- |
+ |
+ |
+ |
+ |
+ |
- |
- |
+ |
+ |
+ |
+ |
O13 |
R |
Note: (+) positive reaction, (-) negative reaction, Ox: oxidase, Cat : catalase, DCA: arginine decarboxylase, DCL: lysine decarboxylase, DCO: ornithine decarboxylase, URE: urease, IND: indole, VP: Voges-Proskauer, Glu: glucose, O-ser: O-serotype, AMX: amoxicilin, R: resistant.
Table 2
Biochemical characters of Aeromonas hydrophila under different stress conditions in comparison to standard conditions.
Caractéristiques biochimiques d’Aeromonas hydrophila en différentes conditions de stress en comparaison aux conditions standard.
|
NO3 |
TRP |
GLU |
ADH |
URE |
ESC |
GEL |
PNG |
GLU |
ARA |
MNE |
MAN |
NAG |
MAL |
GNT |
CAP |
ADI |
MLT |
CIT |
PAC |
OX |
CAT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Species |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S0 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
- |
- |
+ |
+ |
+ |
+ |
+ |
S1 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
S2 |
+ |
- |
- |
- |
- |
+ |
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
- |
+ |
+ |
+ |
- |
+ |
+ |
Note : (+) positive response; (-) : negative response. NO3 : nitrate reductase; TRP: tryptophane desaminase; GLU: glucose acidification; ADH: arginine dihydrolase; URE: urease; ESC: esculine hydrolyases; GEL: gelatinase; PNPG: p-nitrophenyl-ß-galactopyranoside; Glu: glucose assimilation; ARA: arabinose; MNE: mannose; MAN: mannitol; NAG: N-acetyl-glucosamine; MAL: maltose; GNT: gluconate; CAP: caprate ; ADI: adipate; MLT: malate; CIT: citrate; PAC: phenyl-acetate; OX: oxidase; CAT: catalase.
Table 3
Modifications of antibiotic profiles of the Aeromonas hydrophila strains after stress conditions.
Modifications des profils antibiotypiques de la souche d’Aeromonas hydrophila après le séjour en conditions de stress.
Strains |
Decreasual susceptibility |
Antibiotics* |
|---|---|---|
S0 |
6 |
Amx –Ox-S-FM -RA-Nov |
S1 |
4 |
Amx –Ox-FM-Nov |
S2 |
9 |
Amx –Ox-S-FM-RA-Nov-C-SXT-AR |
(*) Different antibiotics: AMX: amoxicillin; OX: oxacillin; S: streptomycin; FM: furans; RA: rifampicin; Nov: novobiocin; C: chloramphenicol; SXT: trimethoprim-sulfamethoxazole ; AR: oxalinic acid.