Résumés
Abstract
The discharge of industrial wastewater and domestic sewage from Kara city affects the quality of Kara river water. To mitigate this water pollution, the capacity of mesoporous charcoal prepared from rice husk (RH) to remove the chemical oxygen demand (COD) in wastewater samples from five sites of Kara City was investigated. The temperature, pH, conductivity, total suspended solids (TSS), biochemical oxygen demand (BOD5), chemical oxygen demand (COD) and oxidizable matters (OM) of samples were analyzed. Batch experiments were applied to study the COD reduction by using powdered RH and two types of activated charcoals (AC). The experimental parameters used to identify optimal conditions for COD abatement are solution pH, contact time, mass of adsorbent and initial value of COD including the nature of wastewater. Activated charcoals showed a higher attenuation capacity of the COD in comparison with the rice husk powder. Maximal abatement rate (100%) of COD removal was obtained for the wastewater sample with a COD of 1 060 mg O2∙L-1 treated at pH 8 with the charcoal GAC-Base using an adsorbent concentration of 10 g∙L-1 with a contact time of 60 min.
Keywords:
- Chemical oxygen demand,
- wastewater,
- rice husk,
- activated charcoal,
- removal,
- chemical activation
Résumé
Les rejets d’eaux usées domestiques et industrielles de la ville de Kara affectent la qualité de l’eau de la rivière Kara. Notre travail porte sur l’évaluation de la capacité du charbon actif mésoporeux préparé par activation chimique à partir de la balle de riz à éliminer la demande chimique en oxygène (DCO) et contribuer ainsi à la réduction de la pollution de la rivière de Kara. Cinq échantillons d’eaux usées prélevés sur cinq sites de la ville de Kara ont été caractérisées par le pH, la température, la conductivité, les matières en suspension (MES), la DCO, la DBO5 et les matières oxydables (MO). Les tests de réduction de la DCO ont été réalisés par la méthode batch avec la poudre de balle de riz et deux types de charbons activés. Les effets du pH du milieu, du temps de contact, de la masse de l’adsorbant, de la valeur initiale de la DCO et la nature de l’eau usée sur la réduction de la DCO ont été évalués. Les charbons activés présentent une capacité d’atténuation de la DCO plus élevée en comparaison avec la poudre de balle de riz pour la réduction de la DCO des eaux usées. Le taux d’abattement maximal de la DCO est obtenu à 100 % pour une eau usée dont la DCO était de 1 060 mg O2∙L-1 traitée à pH 8 avec le charbon CAG-Base à la concentration en adsorbant de 10 g∙L-1 pendant 60 min de contact.
Mots-clés :
- Demande chimique en oxygène,
- eau usée,
- balle de riz,
- charbon activé,
- réduction,
- activation chimique
Corps de l’article
1. Introduction
Dissemination of waste in the Kara city in Togo built in the catchment of Kara river is one of the largest sources of pollution of Kara river (SEGBEYA, 2012). Indeed, the wastes deposited near the river are sources of leachate rich in organic matter. Their drainage to the river contributes to increase organic load of water and sediment that promotes the development of eutrophication of the river. Wastewaters constitute a threat for environment and human health. However, they can be used for many purposes such as irrigation, energy production and sometimes for drinking water production, if one can properly clean it. Several methods including ultrafiltration, reverse osmosis, ion exchange, advanced oxidation processes, adsorption (KORBAHTI et al., 2007; MAHVI et al., 2005; MARTINS and MARTINS, 1993) have been developed to clean a variety of industrial wastewaters. Some of these technologies require addition of chemicals and produce large volume of sludge that requires further treatment before disposal. The implementation of the adsorption process can involve the utilization of several agricultural materials which contain of suitable carbonaceous substances useful to treat water and wastewater.
Adsorption is defined as an interface phenomenon due to the interactions between film particles and adsorbent surface (BOUCHELTA, 2003). It is one of the effective ways to remove color, odor, and organic and inorganic pollutants from wastewaters. Biosorption is an emerging technology which has been successfully investigated in various studies for pollutants removal from water. Organic pollutants are from source such as domestic waste that incorporates domestic, industrial and municipal wastewater. Removal of organic pollutants and heavy metals by adsorption has been widely used for wastewater treatment (DEVI and DAHIYA, 2010; PARANDE et al., 2009). Industrial wastewater often contain appreciable concentrations of biochemical oxygen demand (BOD), chemical oxygen demand (COD), suspended solids, highly toxic compounds and dyes (ABOULHASSAN et al., 2006; AKYOL, 2012; MALAKOOTIAN et al., 2008). The decomposition of organic compounds by micro-organisms in the aquatic ecosystem leads to the reduction of dissolved oxygen level, which in turn inhibits the growth or cause the death of the aquatic habitats (ONUEGBU et al., 2008). In addition, biodegradation products could be toxic. COD represents the quantity of oxygen necessary for total chemical oxidation of oxidizable matters that are organic, inorganic, suspended or dissolved in the water (CREPA, 2007). The oxygen quantity used for the destruction of organic matters decomposable by biochemical processes for five days corresponds to BOD5. COD measures the pollutant charge of a sample in organic matters (CREPA, 2007). Its chemical composition is linked to the oxygen quantity needful for the oxidation of a few matters in the water by potassium dichromate.
Activated charcoals (AC) have high surface area, porosity and high adsorption capacity (AVOM et al., 2001; THAJEEL et al., 2013). Activated charcoals are well known as an alternative to biological and physical-chemical methods in wastewater treatment due to its good adsorptive ability (ISMADJI and BHATIA, 2001).They have been used for the treatment of polluted waters reducing cost with simply and efficiency technology (SULAYMON et al., 2010). In addition, they are used to reduce COD and BOD from a variety of industrial wastewaters (AMUDA and IBRAHIM, 2006; DEVI and DAHIYA, 2010).
Despite the usefulness of AC as adsorbent in wastewater treatment, its use has been restricted due to costly maintenance (EL-GEUNDI, 1997). Studies have been focused on the preparation of new composite adsorbents with low-cost materials that have the additional virtue of possessing improved adsorptive properties compared to other conventional adsorbents (HALIM et al., 2009; LEBODA, 1993; GAO et al., 2005). Rice husk yields a high ash content of 19% (wt) mainly consists of silica and carbon (APICHAT and EAKACHAI, 2010). Typical chemical composition of rice husk (RH) ash is: 84.3% SiO2, 12.2% loss on ignition (mainly carbon materials), 1.4% CaO, 0.6% Fe2O3, 0.5% MgO, 0.4% Na2O, 0.3% Al2O3, and 0.2% K2O3 (NAIYA et al., 2009).
Since the main components of RH are carbon and silica, it has the potential to be used as a raw material in composite adsorbent for both organic and inorganic pollutant removal. Many studies have been done on COD removal using leachate of RH (HALIM et al., 2011) chemical AC from RH (VAN and THI, 2014; MUKUNDAN and RATNOJI, 2015; DAFFALLA et al., 2010).
It is well known that activated charcoals are effective adsorbents for the removal of organic pollutants from aqueous or gaseous phase. Therefore, this type of adsorbent finds wide application as a commercial adsorbent in the purification of water. Many researches were focused on modifying AC surfaces, or on producing composite adsorbents that have the ability to interact with both organic and inorganic adsorbates. The structure of AC from RH can be microporous (DAFFALLA et al., 2010) or totally porous with cracks and crevices (VAN and THI, 2014).
In this work, we aimed to investigate the removal of COD from wastewater using the agricultural by-products as rice husk (RH) regarding the effect of various operating conditions on COD abatement. The RH got interest as it is an agricultural by-product, non-toxic, bio-degradable, abundant and renewable. It has been used by some authors for heavy metals removal and COD removal in polluted water (EL-DARS et al., 2013; OLIVEIRA et al., 2005).
Batch adsorption equilibrium experiments were carried out to determine the optimal conditions for COD removal in the investigated wastewater using two types of activated charcoal. The abatement capacity of charcoal was compared to powder RH (PRH). Effects of contact time, initial pH, adsorbent dose and initial COD concentration of the wastewater were investigated.
2. Experimental section
2.1. Activated charcoal preparation
RH was obtained from rice production fields at KOUBRI Village in Burkina Faso. RH was washed thoroughly to remove any dirt and then dried in a stove at 105 °C during 2 h. The PRH was obtained by crushing the dried RH in powdery particles size. Chemical activation of the dried RH was done using 0.16 N NaOH (denoted as GAC-Base hereafter) or 0.2 N H3PO4 (denoted as GAC-Acid hereafter) according to the method described by THAJEEL et al. (2013). Each activated RH was filtered and dried in an oven at 120 °C for 4 h. The carbonization of dried activated RH was carried in an oven at 650 °C for 2 h using a heating speed of 30 °C∙min-1.
2.2. Charcoal characterization
For the determination of charcoals characteristics, Brunauer-Emmett-Teller (BET) experiments were used using BET surface analyzer at liquid nitrogen temperature. Elemental composition of charcoals was analyzed with energy dispersive spectroscopy (EDS) while scanning electron microscopy (SEM) was used to determine the surface morphology of charcoal particles. The point of zero charge (pHZPC) was determined according to the method described by NOH and SCHWARZ (1989) and bulk density by using the volumetric method described by LASKA (2005). Fourier Transform-Infrared spectra of charcoal material were recorded to detect the surface functional groups using an infrared spectrophotometer (Tensor 27, Bruker, Germany) operating in the range of 4 000-400 cm-1 and employing the potassium bromate pellet (KBr) method.
2.3. Wastewater sampling and analysis
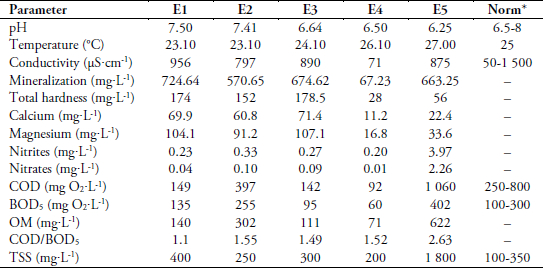
Wastewater samples were collected from five sites namely E1, E2, E3, E4 and E5 (Table 1) in Kara city (Togo) on 29 October and 24 November 2014. Wastewater samples were filtrated immediately after sampling and total suspended solids (TSS) determined. Afterward, samples were stored at 4 °C with a drop of sulfuric acid to avoid any change in their physico-chemical characteristics.
Table 1
GPS data of sampling sites
Coordonnées GPS des sites d’échantillonnage
2.4. Wastewater characterization
The COD of wastewater sample was determined using closed reflux colorimetric method (APHA, 1995) and BOD5 was measured by the manometric method with a respirometer (Oxodirect). The temperature, pH, conductivity and mineralization were measured during the sampling using a pHmeter (InoLab 730, WTW) and a conductimeter (HI 99300, Hanna) while the concentrations of nitrites, nitrates, calcium, magnesium and total hardness were determined using available chemicals in the laboratory by volumetric methods with UV-spectrophotometer (6305, Jenway).
2.5. COD removal experiments
The reduction experiments of COD with charcoals were carried out in batch mode. 50 ml of wastewater with known COD value were stirred in a 250 mL Erlenmeyer at 200 rpm with various weights of adsorbent material ranging from 0.1 to 0.5 g, at various mixing time (15-90 min) and throughout pH range of 5-8 at 25 ± 2 °C. The treated sample was then filtered using Whatman filter. Each experiment was carried out three times and the average value was used for the calculations.
where C0 and Ce are the initial and equilibrium concentrations of COD, respectively.
The COD/BOD5 ratio was used as indicator of the samples biodegradability. The effect of some parameters such as initial pH, contact time, initial COD value of the sample and adsorbent dose affecting the removal efficiency was studied. The effect of pH on COD removal was studied at two values: 5 and 8. pH was adjusted at these desired value with 0.1 M HCl and 0.1 M NaOH solutions. PRH is chosen as reference; therefore, pH value with maximum COD removal using PRH will be used as optimum pH for follow up experiment in the study.
Optimum contact time was determined by varying the contact time from 15 to 90 min. Each experiment was conducted with 2 g∙L-1 of adsorbate in contact with wastewater sample. To find out the optimum dose of adsorbent on COD removal, experiments were carried out using various adsorbent mass in the range of 0.1-0.5 g of activated charcoal. COD removal on PRH and AC from RH is influenced by initial COD concentration and the study was conducted at initial values of COD ranged from 92 to 1 060 mg O2∙L-1.
3. Results and discussion
3.1. Adsorbent characteristics
Elemental composition (%, wt/wt) of charcoals is given in Table 2. Both charcoals contain mostly carbon (C), silicon (Si) with the presence of sodium (Na) in charcoal GAC-Base only vs to GAC-Acid. GAC-Acid has high carbon rate compared to GAC-Base which is richer in silicon. GAC-Base has higher ash rate comparatively to GAC-Acid. The high silicon rate combined with the low carbon content in GAC-Base is attributable to the conversion of carbon to ash in the carbonization process comparatively to GAC-Acid. This result is in agreement with works of GUEYE (2015) which concluded the low cellulose rate and high ash rate in GAC-Base comparatively to GAC-Acid. The form of silica in AC couldn’t be determined exactly but it can be silicon oxide (SiO2) in ash content. VAN and THI (2014) have found only 83.04% C and 16.96% O in the chemical composition of AC from RH. The decrease of oxygen rate in activated charcoals compared to raw RH material is attributable to the breaking of oxygen bond in cellulose, hemicellulose and lignin structures during the carbonization. In addition, most of oxygen rate is concentrated in the ash rate which is soluble in water and removed after washing of activated charcoals.
Table 2
Elemental composition of charcoals
Composition élémentaire des charbons
Energy dispersive spectroscopy spectra are presented in Figures 1a and 1b for GAC-Acid and GAC-Base, respectively. Rapid comparison of Figures 1a and 1b highlighted the absence of sodium in GAC-Acid. The presence of sodium in the composition of GAC-Base can be due to a low interaction with traces of NaOH on the inorganic matter of raw RH that contains 5.5% of the following mixture oxides: CaO, Fe2O3, MgO, Al2O3, Na2O, K2O, MnO2 (CHOWDHURY et al., 2011). In addition, GAC-Base contains a high ash while the ash of RH contains 0.4% of Na2O (NAIYA et al., 2009).
Figure 1
Energy Dispersive Spectrum of a) GAC-Acid and b) GAC-Base
Spectre à dispersion d’énergie du charbon : a) CAG-Acide et b) CAG-Base
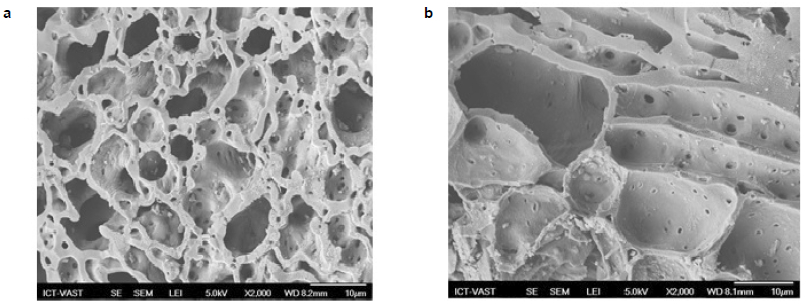
Figures 2a and 2b show the scanning electron microscopy magnified 2 000 times of charcoals GAC-Acid and GAC-Base, respectively. SEM micrographs of activated charcoals show that they have averagely porous structures with regular mesopores. The holes of GAC-Acid indicate that the external surfaces have many cavities which are suitable for adsorption to occur comparatively to GAC-Base where the holes are larger. Similar structure of AC from RH was found by SALAME and BANDOSZ (2000). All AC samples have a mesoporous structure with cracks and crevices. Previous to COD removal, AC particles have large cavities indicating the high possibility of absorption. As shown by AC micrographs, the pores were developed from the decomposition of raw RH structure by heating and converted it to small particles with large surface area. The chemical activation affects significantly the development of surface area and the evolution of pore structure (DAFFALLA et al., 2010).
Figure 2
Scanning Electron Microscopy at 2 000 magnification of a) GAC-Acid and b) GAC-Base
Microscopie électronique à balayage du charbon avec grossissement de 2 000 : a) CAG-Acide et b) CAG-Base
The characteristics of the prepared charcoals are provided in Table 3. Data reported in this table showed that the two charcoals are mesoporous and have similar pore diameter while GAC-Acid has a higher surface area and pore volume. According to GUEYE (2015), the high surface area of GAC-Acid is due to the high rate of cellulose in the RH favoring activation by phosphoric acid. In opposite, the low surface area of GAC-Base can be explained by the fact that the RH is poor in carbon rate (GUEYE, 2015). These results are in agreement with effects observed by THAJEEL et al. (2013) who found a higher BET surface for GAC-Acid compared to the one of GAC-Base. Excepted for the pH of zero point charge (pHZPC), all characteristics of the activated charcoals such as surface area and pore volume are lower than those of AC prepared by THAJEEL et al. (2013) using the same chemical process. This can be due to the fact that the washing of activated RH has been done after the carbonization, not before the carbonization. The dried RH with traces of activation agent causes the decrease of activated RH dimensions (surface area, pore volume). Moreover, the increase of activation agent concentration causes an increase of surface area and pore volume while pHZPC stayed nearly the same (MUKUNDAN and RATNOJI, 2015).
Table 3
Characteristics of charcoals
Caractéristiques des charbons
FT-IR spectra in the range 4 000-400 cm-1 of charcoals are shown in Figures 3a and 3b for GAC-Acid and GAC-Base, respectively. For GAC-Acid spectrum, the band around 464 cm-1 is attributed of Si-O bond stretching while the bond Al-O was observed at 797 cm-1. The band at 1 094 cm-1 corresponds to the C-O bond and the band at 1 585.6 cm-1 was attributed to C = C bond stretching. An alcohol hydroxyl group at 3 420.3 cm-1 was observed as well shown in Figure 3a. In the case of GAC-Base, in addition of bands observed for GAC-Acid, it was observed a band around 793 cm-1 attributable to Na-O bond stretching. These spectra suggest the presence of quartz, alumina, which can affect the development of the adsorbent pores, and surface functions such as lactone and phenolic compounds.
Figure 3
FT-IR spectrum of a) GAC-Acid and b) GAC-Base
Spectre TF- infrarouge du charbon : a) CAG-Acide et b) CAG-Base
FT-IR spectra and EDS observations correlate with the composition of raw RH that contains 74.5% organic matter which consists of about (32% cellulose, 21% hemicelluloses, 21% lignin, 20% silica and 3% crude protein), and the rest is inorganic matter containing 20% SiO2 and 5.5% of the following mixture oxides: CaO, Fe2O3, MgO, Al2O3, Na2O, K2O, MnO2, as well as traces of Cu and Pb (CHOWDHURY et al., 2011; MARKOVSKA and LYUBCHEV, 2007).
3.2. Classification of the wastewater
The data obtained for temperature, pH, conductivity, mineralization, concentrations of nitrites and nitrates are given in Table 4 and compared to the Ireland Environmental Protection Agency (IEPA) norms of urban wastewaters (IEPA, 1997). All samples have more nitrites than nitrates in the wastewater. The initial values of COD, BOD5, OM (oxidizable matters) and TSS of sample are shown in Table 4. COD/BOD5 ratio values of the samples are less than 3, indicating that all samples are biodegradable (DHOUIB et al., 2005). However, the high value of ratio 2.63 for the sample E5 (University restaurant) highlighted that this sample is hardly biodegradable. This can be explained by the origin of this wastewater rich in organic nutrients as food wastes. In addition, with high rate of TSS, the oxidation will be difficult by biochemical processes, resulting to the decrease of BOD5. Then, it could be due to lumps of freshly discarded foods in the wastewater and that are biodegradable. All contributes to increase the value of COD/BOD5 ratio in this sample E5.
Table 4
Values of physical-chemical parameters of wastewater samples before treatment
Valeurs des paramètres physicochimiques des échantillons avant traitement
3.3. COD removal
3.3.1. Effect of initial pH
Two values of pH are chosen for experiments: an acid pH of 5 and a basic pH of 8. It is known that pH of solution is an important parameter that affects the properties of adsorbate and adsorbent as well as the adsorption process in aqueous solutions (NJOKU and HAMEED, 2011).The influence of initial pH on COD removal in wastewater was evaluated at pH 5 and pH 8 using 2 g∙L-1 of PRH, GAC-Acid and GAC-Base The results obtained by using activated charcoals and PRH are illustrated in Table 5.
Table 5
Effect of pH on COD removal using PRH and charcoals (2 g∙L-1) for 60 min of contact time
Effet du pH sur l’abattement de la DCO utilisant 2 g∙L-1 d’adsorbants (PRH et charbons activés) pour 60 min de contact
The COD removal from wastewater (E3) is higher in acid medium with activated charcoals while it is higher with PRH in basic medium as an indication that pH has a significant effect on COD removal. All percentages of COD removal using activated charcoals were higher in acid medium compared to PRH due to the high surface area of charcoals. GAC-Base charcoal with low surface area is less efficient in COD removal at high and average concentrations of COD. Then, we noticed that the rate of COD removal decreases when pH of solution increases with both activated charcoals as adsorbent. This is attributable to the electrostatic interaction between the adsorbed compounds and the adsorbent surface (EL-NAAS et al., 2010). Then, pH of zero point charge (pHZPC) of both activated charcoals allows the distribution of the elemental charge on the adsorbent surface as depending on pH of solution. Optimum pH (pH 8) obtained with PRH was used in following experiments and this pH is high than pHZPC of both charcoals. This indicates that the surface charge of GAC-Acid is negative and if the adsorption has occurred by electrostatic fixation, it concerns mainly the positively charged compounds. But the surface of GAC-Base is practically neutral at pH 8 (pH 8 is nearest of his pHZPC that is 7.94). Hence, others mechanisms such as precipitation can involve the removal of anions (nitrites and nitrates). The powder of raw rice husk (PRH) was taken as reference and charcoals were compared to its in order to know the effect of chemicals in charcoal efficiency for COD removal. Indeed, chemicals (NaOH and H3PO4) allow the development of surface area and pore size of charcoals. In addition, chemical activation reduces the content of hemicellulose, lignin and cellulose crystallinity which leads to an increase of specific surface area for treated RH compared to raw RH (DAFFALLA et al., 2010). This causes a high adsorption capacity of COD on activated charcoals.
3.3.2. Effect of contact time
The Figure 4 shows the effect of contact time on COD removal. Using the activated charcoals, the percentage of COD removal increases when the contact time increases with an optimal reduction time ranged between 60-90 min (Figure 4). These results are within the range of equilibrium times reported for the reduction of COD in some industrial effluents using commercial AC (DEVI et al., 2008) and activated RH (CHOWDHURY et al., 2011). Optimum removal was achieved 60 min of contact time with a removal percentage of 92% for GAC-Acid and 46% for GAC-Base. COD removal is increased for PRH up to 90 min (Figure 4). According to experimental data and activated charcoals characteristics (surface area, mesoporous structure, and pore size distribution), adsorption on monolayer and surface mass transfer are likely the process occurring with the three adsorbent materials. Similar result has been found by EL-DARS et al. (2013). The literature data indicate that intra-particle diffusion is involved in the current process of pollutants adsorption and COD reduction (EL-NAAS et al., 2010). For confirmation purpose, Weber’s intra-particle model was applied using Equation 2:
where q represents the quantity of COD adsorbed, t is the contact time, kd is the intra-particle diffusion rate constant and θ is the intercept.
Figure 4
Effect of contact time on COD removal using activated charcoals and PRH (2 g∙L-1) with an initial value C0 = 1 060 mg O2∙L-1, 50 mL of sample at pH 8
Effet du temps de contact sur la réduction de la DCO en utilisant 2 g∙L-1 de charbons actifs et PRH, C0 = 1 060 mg O2∙L-1, V = 50 mL et pH = 8
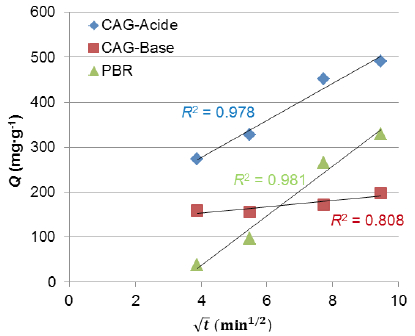
Figure 5 shows the trend of qvs![]() . Correlation coefficients (R2) were found to be 0.978, 0.808 and 0.981 for GAC-Acid, GAC-Base and PRH respectively, and corresponding values of intra-particle diffusion rate constant were 43.97, 7.08 and 54.86 mg∙g-1∙min-1, respectively. The resultant between the intra-particle diffusion and film diffusion was the controlled step of adsorption process using GAC-Acid and PRH, as the linear portion of curve doesn’t pass through the origin.
. Correlation coefficients (R2) were found to be 0.978, 0.808 and 0.981 for GAC-Acid, GAC-Base and PRH respectively, and corresponding values of intra-particle diffusion rate constant were 43.97, 7.08 and 54.86 mg∙g-1∙min-1, respectively. The resultant between the intra-particle diffusion and film diffusion was the controlled step of adsorption process using GAC-Acid and PRH, as the linear portion of curve doesn’t pass through the origin.
Figure 5
Curve of intra-particle diffusion
Courbe de la diffusion intra-particulaire
The low correlation coefficient and the non-linear curve found with GAC-Base, indicate that the film diffusion remains the main step of adsorption process. Increase in COD uptake from 0 to 60 min is a consequence of the solute adsorption onto the large available surface of the adsorbent particles until surface saturation was reached (AHMAD and HAMEED, 2009). Afterward, molecules require more time to diffuse through the pores to reach the interior surface of the particles.
From this result, 60 min of contact time was used for all experiments. Previous works (AZBAR et al., 2004) showed similar results using advanced oxidation processes (AOPs) and chemical treatment methods for COD removal in effluents. Result of the above mentioned work, indicate that the percentage of COD reduction increases with a contact time. In our study, we noticed a slight variation of time in COD removal from 60 to 90 min of contact time with GAC-Base indicating that PRH is more efficient in COD removal than GAC-Base for time over 60 min.
3.3.3. Effect of initial value of COD
The effect of initial COD value on COD removal is illustrated in Figure 6. COD removal increases when the initial value of COD increases from 92 to 1 060 mg O2∙L-1 using PRH and GAC-Acid (Figure 6). High removal rates of COD (96% and 75.31%) were obtained from respectively for 397 and 1 060 mg O2∙L-1 (E2 and E5). This increase of COD removal rate can be the result of free sites occupation, inaccessible on adsorbent surface at low concentrations of COD (TAZEROUTI and AMRANI, 2009). However using GAC-Base, the percentage of COD removal decreases with the initial concentration of COD increased and high removal (73.35%) was observed at low initial value 92 mg O2∙L-1. This can be explained by the low surface area and high ash rate in GAC-Base and a high quantity of COD can cause desorption of adsorbed molecules on GAC-Base surface. Only sample E4 (92 mg O2∙L-1) had a high COD reduction using GAC-Base of 73.35%, which could be linked to the low concentration of nitrate, nitrite, total hardness and COD in this sample highlighting the importance of initial statue of sample in term of some compounds concentration. Except the case of sample E3 (142 mg O2∙L-1) where all adsorbents seem equally performant in COD removal, GAC-Base had opposite trend variation compared to two others adsorbents (GAC-Acid and PRH). Wastewater with high values of COD is better treated with GAC-acid or PRH because the removal effectiveness increases with COD charge. GAC-Base remained only efficient at low concentrations of COD. These results could be explained by the complementary effect of surface functions groups and adsorbent properties (mesoporosity, pores volume, surface area, pHZPC).
Figure 6
Effect of initial COD value on COD removal using 2 g∙L-1 of adsorbent for 60 min of contact time, 50 mL of sample at pH 8
Effet de la valeur initiale de la DCO sur sa réduction en utilisant 2 g∙L-1 de charbon et PRH pour 60 min de contact, V= 50 mL et pH = 8
3.3.4. Effect of adsorbent quantity
The Figure 7 shows the effect of adsorbent mass on COD removal. The wastewater sample E4 with initial value COD value of 92 mg O2∙L-1 (lower value of COD compared to the others samples) is used for this study. When the quantity of activated charcoal increases from 0.1 to 0.5 g, the percentage of COD removal increases from 18.1% to 55.63% for GAC-Acid and from 73.35% to 100% for GAC-Base (Figure 7). The initial quantity of activated charcoal selected for previous experiments was 0.10 g and the sample E4 was used to evaluate the effect of adsorbent mass on the COD removal rate. This increase of COD removal rate was attributed to the availability of solute and the increase of available sites number adsorbing the pollutants (nitrites, calcium, magnesium and nitrates) which can submit a chemical oxidation. Previous studies have shown that the application of commercial AC to wastewaters achieved over 90% removal of the non-biodegradable and biodegradable content in wastewater (DEVI and DAHIYA, 2010). It was also noted that the increase of the adsorbent weight beyond the optimum amount (10 g∙L-1) in this study was not correlate to the percentage of COD removal for GAC-Base which remains constant thereafter. The value (2 g∙L-1) used in experiments above was a minimum value to test the efficiency of AC in COD removal. These various mass reflect the variation in surface area obtained for the prepared activated materials that indicated GAC-Acid had a relatively larger surface area than GAC-Base. In addition, the change in the observed adsorption capacities among of two adsorbent materials may be attributed to the difference in number of carbonaceous adsorption sites available on each charcoal (DEVI, 2010). Moreover, this increase of COD removal percentage can be explained based on increase in number of pores/active sites as well as surface area with an increase in the amount of adsorbent (DEVI et al., 2008).
Figure 7
Effect of adsorbent mass on COD removal for 60 min of contact time, 50 mL of sample and initial value C0= 92 mg O2∙L-1 at pH 8
Effet de la quantité de l’adsorbant sur la réduction de la DCO pour 60 min de contact, V = 50 mL, C0 = 92 mg O2∙L-1 et pH = 8
4. Conclusion
In this present study, adsorbents such as PRH, GAC-Acid and GAC-Base were found to be effective adsorbents for the removal of COD in wastewaters. The results indicated that a complete removal of COD (100%) was achieved at pH 8 for 60 min using 10 g∙L-1 of GAC-Base at low concentrations of COD (92 mg O2∙L-1) only. With high concentrations of COD, GAC-Acid and PRH were best efficient adsorbents with removal of 92.55% and 95.47%, respectively. The removal of COD depends clearly on the water sample characteristics, contact time, pH of mixture and adsorbent quantity. Optimal conditions obtained in our experiment for best removal of COD are an average initial COD value of 300 mg O2∙L-1with 10 g∙L-1 of adsorbent at pH 8 for 60 min of contact time. In conclusion, activated charcoals were best adsorbents compared to PRH for COD removal and GAC-Acid was a best adsorbent among three tried adsorbents. 10 g∙L-1 of adsorbent dose is recommended for a maximum removal of COD in Kara river wastewater.
Parties annexes
Acknowledgements
This work was supported financially by Excellence Center for Development Cooperation (EXCEED), Federal Ministry of Economic Cooperation Development-Germany (BMZ), German Academic Exchange Service (DAAD), and Technical University of Braunschweig (TUBS).
Références
- ABOULHASSAN M.A., S. SOUABI, A. YAACOUBI and M. BAUDU (2006). Improvement of paint effluents coagulation using natural and synthetic coagulant aids. J. Hazard. Mater., 138, 40-45.
- AHMAD A.A. and B.H. HAMEED (2009). Reduction of COD and color of dyeing effluents from a cotton textile mill by adsorption onto bamboo-based activated carbon. J. Hazard. Mater., 172, 1538-1543.
- AKYOL A. (2012). Treatment of paint manufacturing wastewater by electrocoagulation. Desalination, 28, 91-99.
- AMERICAN PUBLIC HEALTH ASSOCIATION (APHA) (1995). Standard Methods for Examination of Water and Wastewater. APHA 19th edition, Washington DC, USA, 1282 p.
- AMUDA O.S. and A.O. IBRAHIM (2006). Industrial wastewater treatment using natural material as adsorbent. Afr. J. Biotechnol., 5 (16), 1483-1487.
- APICHAT I. and P. EAKACHAI (2010). Humic acids removal from water by aminopropyl functionalized rice husk ash. J. Hazard. Mater., 184 (1-3), 775-781.
- AVOM J., J. KETCHA MBADCAM, M.R.L. MATIP and P. GERMAIN (2001). Adsorption isotherme de l’acide acétique par les charbons d’origine végétale. Afr. J. Sci. Technol., 2 (2), 1-7.
- AZBAR N., T. YONAR and K. KESTIOGLU (2004). Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent. Chemosphere, 55, 35-43.
- BOUCHELTA C. (2003). Étude de l’adsorption des métaux Hg2+,Cu2+, Zn2+, Fe3+, Cr6+ sur le charbon actif en grain : modélisation. Magister Thesis, Univ. Badji Mokhtar-Annaba, Algeria, 169 p.
- CENTRE RÉGIONAL POUR L’EAU POTABLE ET L’ASSAINISSEMENT À FAIBLE COUT (2007). Contrôle et suivi de la qualité des eaux usées : protocole de détermination des paramètres physico-chimiques et bactériologiques. Centre collaborant de l’OMS, Burkina Faso, 52 p.
- CHOWDHURY S., R. MISHRA, P. SAHA and P. KUSHWAHA (2011). Adsorption thermodynamics, kinetics and esoteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination, 265, 159-168.
- DAFFALLA S.B., H. MUKHTAR and M.S. SHAHARUN (2010). Characterization of adsorbent developed from rice husk: effect of surface functional group on phenol adsorption. J. Appl. Sci., 10 (12), 1060-1067.
- DEVI R. (2010). Innovative technology of COD and BOD reduction from coffee processing wastewater using avocado seed carbon (ASC). Water Air Soil Pollut., 207, 299-306.
- DEVI R. and R.P. DAHIYA (2010). COD and BOD removal from domestic wastewater generated in decentralized sectors. Bioresour. Technol., 99, 344-349.
- DEVI R., V. SINGH and A. KUMAR (2008). COD and BOD reduction from coffee processing wastewater using Avocado peel carbon. Bioresour. Technol., 99, 1853-1860.
- DHOUIB A., M. ELLOUZ, F. ALOUI and S. SAYADI (2005). Effect of bioaugmentation of activated sludge with white-rot fungi on olive mill wastewater detoxification. Lett. Appl. Microbiol., 42 (4), 405-11.
- EL-DARS F.M.S.E., M.A. IBRAHIM and M.E.G. ADEL (2013). Reduction of COD in water-based paint wastewater using three types of activated carbon. Desalin. Water Treat., 52 (16-18), 2975-2986.
- EL-GEUNDI M.S. (1997). Adsorbents of industrial pollution control. Adsorpt. Sci. Technol., 15, 777-787.
- EL-NAAS M.H., S. AL-ZUHAIR and M. ABU ALHAIJA (2010). Reduction of COD in refinery wastewater through adsorption on date-pit activated carbon. J. Hazard. Mater., 173, 750-757.
- GAO N.F., S. KUME and K. WATARI (2005). Zeolite-carbon composites prepared from industrial wastes: (II) evaluation of the adaptability as environmental materials. Mat. Sci. Eng. A-Struct., 404 (2), 274-280.
- GUEYE M. (2015). Développement de charbon actif à partir de biomasses lignocellulosiques pour des applications dans le traitement de l’eau. PhD Thesis, Institut International de l’Ingénierie de l’Eau et de l’Environnement, Burkina Faso, 229 p.
- HALIM A.A., H.A. AZIZ, M.A.M. JOHARI and K.S. ARIFFIN (2009). Removal of ammoniacal nitrogen and COD from semi-aerobic landfill leachate using low cost activated carbon-zeolite composite adsorbent. Int. J. Environ. Waste Manag., 4 (4), 399-411.
- HALIM A.A., N.N.Z. ABIDIN, N. AWANG, A. ITHNIN, M.S. OTHMAN and M.I. WAHAB (2011). Ammonia and COD removal from synthetic leachate using rice husk composite adsorbent. J. Urban Environ. Eng., 5, 24-31.
- IRELAND ENVIRONMENTAL PROTECTION AGENCY (IEPA) (1997). Waste water treatment manuals primary, secondary and tertiary treatment. Ireland Environmental Protection Agency, Ardcavan, Wexford, Ireland, 131 p.
- ISMADJI S. and S.K. BHATIA (2001). Characterization of activated carbons using liquid phase adsorption. Carbon, 39 (8), 1237-1250.
- KORBAHTI B.K., N. AKTAS and A. TANYOLAC (2007). Optimization of electrochemical treatment of industrial paint wastewater with response surface methodology. J. Hazard. Mater., 148, 83-90.
- LASKA M. (2005). An evaluation of fresh water mollusk shell sand as an adsorptive media for removing phosphorus in a constructed wetland filter. Master Thesis, Queen’s University, Canada, 176 p.
- LEBODA R. (1993). Carbon-mineral adsorbents - new type of sorbents part II. Surface properties and methods of their modification. Mater. Chem. Phys., 34 (2), 123-141.
- MAHVI A.H., D. NAGHIPOUR, F. VAEZI and S. NAZMARA (2005). Tea waste as an adsorbent for heavy metal removal from industrial wastewaters. Am. J. Appl. Sci., 2, 372-375.
- MALAKOOTIAN M., A. ALMASLI and H. HOSSAINI (2008). Lead and cobalt removal from paint industries effluent using wood ash. Int. J. Environ. Sci. Technol., 5 (2), 217-222.
- MARKOVSKA I.G. and L.A. LYUBCHEV (2007). A study on the thermal destruction of rice husk in air and nitrogen atmosphere. J. Therm. Anal. Calorim., 89 (3), 809-814.
- MARTINS J.E. and T.E. MARTINS (1993). Technologies for Small Water and Wastewater Systems. Van Nostrand Reinhold Company, New York, USA, 366 p.
- MUKUNDAN U. and S.S. RATNOJI (2015). COD removal from sewage by activated carbon from rice husk - An agricultural by product. Int. J. Innov. Res. Sci., Eng. Technol., 4 (6), 5003-5007.
- NAIYA T.K., A.K. BHATTACHARYA, S. MANDAL and S.K. DAS (2009). The sorption of lead(II) ions on rice husk ash. J. Hazard. Mater., 163 (3), 1254-1264.
- NJOKU V.O. and B.H. HAMEED (2011). Preparation and characterization of activated carbon from corncob by chemical activation with H3PO4 for 2,4-dichlorophenoxyacetic acid adsorption. Chem. Eng. J., 173, 391-399.
- NOH J.S. and J.A. SCHWARZ (1989). Estimation of the point of zero charge of simple oxides by mass titration. J. Colloid Interface Sci., 130 (1), 130-157.
- OLIVEIRA E.A., S.F. MONTANBER, A.D. ANDRADE, J.A. NOBREGA and M.E. ROLLEMBERG (2005). Equilibrium studies for the sorption of chromium and nickel from aqueous solutions using raw rice bran. Process Biochem., 40, 3485-3490.
- ONUEGBU T.U., L.O. OKOYE, I.J. DIOHA, P.A.C. OKOYE and P.M. NWAKO (2008). Treated effluents and sludge samples. J. Chem. Soc. Nig., 33 (1), 6-9.
- PARANDE A.K., A. SIVASHANMUGAM, H. BEULAH and N. PALANISW-AMY (2009). Performance evaluation of low cost adsorbents in reduction of COD in sugar industrial effluent. J. Hazard. Mater., 168, 800-805.
- SALAME I. and T.J. BANDOSZ (2000). Effect of surface chemistry and pore structure on adsorption of water and methanol on activated carbons. In: Adsorption Science and Technology: Proceedings of the Second Pacific Basin Conference on Adsorption Science and Technology. DO D.D. (Editor), World Scientific Publishing Co., Singapore, China, pp. 61-65.
- SEGBEYA K.N. (2012). Évaluation de l’impact des déchets ménagers de la ville de Kara (Togo) sur la qualité de la rivière Kara. PhD Thesis, Univ. Limoges and Univ. Kara, France, 226 p.
- SULAYMON A.H. and H.M. ABDUL-HAMEED (2010). A comparative study between Adam-Bohart and Herkins-Jura 4-11 models for adsorption of lead(II) from simulated wastewater by activated carbon. Inter. J. Sci. Technol., 5 (2), 4-11.
- TAZEROUTI N. and M. AMRANI (2009). Adsorption du Chrome (VI) sur la lignine activée. Rev. Sci. Eau, 23 (3), 233-245.
- THAJEEL A.S., A.Z. RAHEEM and M.M. AL-FAIZE (2013). Production of activated carbon from local raw materials using physical and chemical preparation methods. J. Chem. Pharm. Res., 5 (4), 251-259.
- VAN K.L. and T.T.L. THI (2014). Activated carbon derived from rice husk by NaOH activation and its application in supercapacitor. Prog. Nat. Sci. Mat. Int., 24, 191-198.
Liste des figures
Figure 1
Energy Dispersive Spectrum of a) GAC-Acid and b) GAC-Base
Spectre à dispersion d’énergie du charbon : a) CAG-Acide et b) CAG-Base
Figure 2
Scanning Electron Microscopy at 2 000 magnification of a) GAC-Acid and b) GAC-Base
Microscopie électronique à balayage du charbon avec grossissement de 2 000 : a) CAG-Acide et b) CAG-Base
Figure 3
FT-IR spectrum of a) GAC-Acid and b) GAC-Base
Spectre TF- infrarouge du charbon : a) CAG-Acide et b) CAG-Base
Figure 4
Effect of contact time on COD removal using activated charcoals and PRH (2 g∙L-1) with an initial value C0 = 1 060 mg O2∙L-1, 50 mL of sample at pH 8
Effet du temps de contact sur la réduction de la DCO en utilisant 2 g∙L-1 de charbons actifs et PRH, C0 = 1 060 mg O2∙L-1, V = 50 mL et pH = 8
Figure 5
Curve of intra-particle diffusion
Courbe de la diffusion intra-particulaire
Figure 6
Effect of initial COD value on COD removal using 2 g∙L-1 of adsorbent for 60 min of contact time, 50 mL of sample at pH 8
Effet de la valeur initiale de la DCO sur sa réduction en utilisant 2 g∙L-1 de charbon et PRH pour 60 min de contact, V= 50 mL et pH = 8
Figure 7
Effect of adsorbent mass on COD removal for 60 min of contact time, 50 mL of sample and initial value C0= 92 mg O2∙L-1 at pH 8
Effet de la quantité de l’adsorbant sur la réduction de la DCO pour 60 min de contact, V = 50 mL, C0 = 92 mg O2∙L-1 et pH = 8
Liste des tableaux
Table 1
GPS data of sampling sites
Coordonnées GPS des sites d’échantillonnage
Table 2
Elemental composition of charcoals
Composition élémentaire des charbons
Table 3
Characteristics of charcoals
Caractéristiques des charbons
Table 4
Values of physical-chemical parameters of wastewater samples before treatment
Valeurs des paramètres physicochimiques des échantillons avant traitement
Table 5
Effect of pH on COD removal using PRH and charcoals (2 g∙L-1) for 60 min of contact time
Effet du pH sur l’abattement de la DCO utilisant 2 g∙L-1 d’adsorbants (PRH et charbons activés) pour 60 min de contact