Abstracts
Abstract
To date, many developing countries such as Iran have almost completely abandoned the idea of decontaminating oil-polluted soils due to the high costs of conventional (physical/chemical) soil remediation methods. Phytoremediation is an emerging green technology that can become a promising solution to the problem of decontaminating hydrocarbon-polluted soils. Screening the capacity of native tolerant plant species to grow on aged, petroleum hydrocarbon-contaminated soils is a key factor for successful phytoremediation. This study investigated the effect of hydrocarbon pollution with an initial concentration of 40 000 ppm on growth characteristics of sorghum (Sorghum bicolor) and common flax (Linum usitatissumum). At the end of the experiment, soil samples in which plant species had grown well were analyzed for total petroleum hydrocarbons (TPHs) removal by GC-FID. Common flax was used for the first time in the history of phytoremediation of oil-contaminated soil. Both species showed promising remediation efficiency in highly contaminated soil; however, petroleum hydrocarbon contamination reduced the growth of the surveyed plants significantly. Sorghum and common flax reduced TPHs concentration by 9500 and 18500 mg kg‑1, respectively, compared with the control treatment.
Keywords:
- Growth parameters,
- hydrocarbon-polluted soil,
- phytoremediation,
- plant
Résumé
À ce jour, plusieurs pays en voie de développement, comme l’Iran, ont presque complètement abandonné l’idée de décontaminer les sols pollués par le pétrole à cause des coûts élevés reliés aux méthodes conventionnelles (physiques/chimiques) de décontamination des sols. La phytoremédiation est une nouvelle technologie verte qui peut s’avérer une solution prometteuse au problème posé par la décontamination des sols pollués par des hydrocarbures. Évaluer la capacité d’espèces indigènes tolérantes à croître sur des sols âgés et pollués par des hydrocarbures de pétrole représente l’une des étapes clé de la phytoremédiation. Au cours de la présente étude, l’effet de la pollution aux hydrocarbures sur les caractéristiques de croissance du sorgho (Sorghum bicolor) et du lin cultivé (Linum usitatissumum) a été évalué à partir d’une concentration initiale de 40 000 ppm. À la fin de d’étude, des échantillons de sols dans lesquels des plantes avaient obtenu un bon taux de croissance ont été analysés à l’aide d’un appareil CG-DIF afin de déterminer les taux d’hydrocarbures pétroliers (THP) totaux enrayés des sols. Le lin cultivé a été utilisé pour la première fois dans l’histoire de la phytoremédiation de sols contaminés par le pétrole. Les deux espèces ont fait preuve d’une efficacité prometteuse dans les sols fortement pollués. Cependant, la pollution par les hydrocarbures de pétrole a réduit de façon significative la croissance des plantes à l’étude. Le sorgho et le lin cultivé ont réduit la concentration en THP de 9 500 et 18 500 mg kg‑1, respectivement, comparativement au traitement témoin.
Mots clés:
- Paramètres de croissance,
- phytoremédiation,
- plante,
- sol contaminé aux hydrocarbures
Article body
Introduction
Soil contamination by petroleum hydrocarbons is one of the world’s most common environmental problems (US EPA 2000). Total petroleum hydrocarbons (TPHs) are one of the most common groups of persistent organic contaminants (Huang et al. 2005). Generally, the accumulation of contaminants in soils can have destructive effects on the environment and human health. Contaminants present in soils can enter the food chain and seriously affect animal and human health (Khan 2005). The relatively high hydrophobicity of petroleum hydrocarbons results in a considerable ability to accumulate in soils and sediments in comparison with aquatic environments (Karthikeyan and Bhandari 2001). Additionally, the high hydrophobicity of these compounds results in their binding to soil and sediment particles, which ultimately leads to a decrease in bioavailability of these contaminants for biological sorption (Luepromchai et al. 2007; Parrish et al. 2005). Therefore, suitable solutions for the removal or control of these soil contaminants must be found. In the past three decades, various physical, chemical and biological remediation technologies, such as thermal treatment, stabilization, solidification and bioremediation, have been invented and used to remove contaminants from soils. Physical and chemical methods are suitable for decontaminating relatively small areas, while they are very expensive to use over large areas such as the ones contaminated by industrial substances, oil products and mining sites (Chekol et al. 2004; Escalante-Espinosa et al. 2005).
To date, many developing countries such as Iran have almost completely abandoned the idea of decontaminating oil-polluted soils due to the high costs of conventional (physical/chemical) soil remediation methods. Phytoremediation is a relatively new, efficient, environmentally-friendly and promising technology for removing many contaminants such as hydrocarbon pollutants. The synergistic cooperation of plant roots and soil micro-organisms promotes the degradation of persistent organic contaminants in phytoremediation. Removal of petroleum hydrocarbons from soil in phytoremediation is often attributed to micro-organisms living in the rhizosphere, under the influence of plant roots (Luepromchai et al. 2007). Microbial communities in planted soils are greater and more active than in unplanted soils (Johnson et al. 2005; Mueller and Shann 2006). Micro-organisms that live in the rhizosphere benefit from the root exudates and plants in return benefit from the metabolic detoxification of potentially toxic compounds brought about by microbial communities. Additionally, plants benefit from the presence of microbial populations through the recycling and solubilisation of mineral nutrients, as well as the increased supply of vitamins, amino acids, auxins, cytokinins and gibberellins that stimulate plant growth (Escalante-Espinosa et al. 2005). One of the advantages of phytoremediation in comparison to other technologies of soil remediation is the fact that the driving force behind phytoremediation is solar energy, which leads to a considerable decrease in the costs of soil remediation. Adding nutrients to soils through fertilization may increase the plant biomass and thus promote pollutant removal (Luepromchai et al. 2007), as considered in the present study.
Many plant species are sensitive to petroleum contaminants (Huang et al. 2004). A 96% reduction in ryegrass biomass after 30 d of growth on soil contaminated with 25 g kg‑1 petroleum hydrocarbons was observed in a phytoremediation study (Tesar et al. 2002). Despite the rather extensive studies that have been carried out worldwide regarding the phytoremediation of oil-polluted soils, some contradictory results have been reported regarding the efficiency and performance of this technology in removing contaminants from soils (Joner et al. 2004). One of the most important factors explaining these results is that phytoremediation is a site-specific remediation method. Hence, selecting and using native plant species that are tolerant to high concentrations of TPHs in soils is a key factor for the success of phytoremediation. Sorghum (Sorghum bicolor (L.) Moench) and common flax (Linum usitatissumum L.) were used for phytoremediation purposes in the present study.
When looking for species to be used in phytoremediation, the selection of tolerant plants from contaminated sites is the first step. Besides tolerance to petroleum hydrocarbons, other important criteria such as a favourable root system and abundance in a given area should be respected. Additionally, native tolerant species should be preferred since they are adapted to the prevailing environmental conditions (Merkl et al. 2004a). Sorghum and some other plant species such as camelthorn (Alhagi camelorum Fisch.) grow wildly near contaminated sites of the Oil Refinery of Tehran. Sorghum was chosen due to its extensive and fibrous root system. In contrast, camelthorn was not selected because it has non-extensive, weak roots. Common flax was also chosen due to its extensive root system as well as for its appropriate tolerance to oil pollution (Adam and Duncan 2002). Phytoremediation of petroleum hydrocarbon-contaminated soil is mainly based on biodegradation in the rhizosphere (Frick et al. 1999); hence, the plant’s root system is one of the most important factors that should be considered. Plants and their roots can also indirectly influence degradation by altering the physical/chemical conditions of the soil (Cunningham 1996), e.g. by increasing aeration and thus providing oxygen for the degradation of contaminants. Sorghum and common flax seeds are abundantly found in most parts of Iran.
The objectives of the present study were (1) to investigate the effect of TPHs on the growth parameters of sorghum and common flax, including germination, shoot height and biomass, and root length and biomass; and (2) to evaluate the phytoremediation potential of these two plant species in highly contaminated, aged soil. To the best of our knowledge, it was the first time in the history of phytoremediation of petroleum hydrocarbon-contaminated soils that common flax was used; however, Adam and Duncan (2002) had previously studied the influence of diesel fuel on flax germination and found promising results. Furthermore, since nutrient addition to soil through fertilization may also increase plant biomass and thus promote pollutant removal (Luepromchai et al. 2007), the effect of three organic fertilizers on plant growth in hydrocarbon-contaminated soil was also evaluated.
Materials and methods
In order to launch the pilot experiment, contaminated soil was provided from the extremely contaminated soils of pond No. 4 of the Oil Refinery of Tehran. The best locations within pond No. 4, i.e. the locations in which the most significant contamination levels were clearly observable, were chose for sampling. Soils were transferred to an experimental plot outside the refinery. Taking into consideration the significant decrease in contamination level and the soil’s considerable colour change from surface to depth, sampling was done from the soil surface down to the deepest area possible, which contained more contamination. Also, at the far end of the right side of the pond, some insignificant contamination could be seen. This point was not chosen for sampling. Soil samples were ground in order to crush the clods. The soil was then mixed thoroughly and sieved through a 10 mm sieve to remove stones and debris in order to obtain a homogeneous mixture. In most studies, soil is sieved through a 2 mm sieve which, according to AASHTO and Massachusetts Institute of Technology standards, represents the limit between sand and gravel particles (Tahooni 2000). However, this leads to a considerable loss in coarse grain proportion of real soil and to a lack of concordance between real soil from contaminated sites and soil used in phytoremediation experiment. Therefore, in order for the study’s soil structure to reflect the genuine soil structure of the contaminated area, a 10 mm mesh was used. Some physical and chemical properties of the experimental soil are presented in Table 1.
Table 1
Physical and chemical characteristics of the experimental soil used in the phytoremediation study
Parameter |
Value |
Analytical method |
|---|---|---|
Clay (%) |
33 |
Hydrometer measurement |
Silt (%) |
33 |
Hydrometer measurement |
Sand (%) |
20 |
Hydrometer measurement |
Gravel (%) |
14 |
Sieve |
Organic matter (%) |
6.92 |
Walkley-Black |
Organic C (%) |
4.02 |
– |
Soil pH |
7.6 |
1:1 soil/water slurry |
Electrical conductivity (dS m-1) |
2.93 |
1:2 soil/water slurry |
Total N (%) |
0.13 |
Kjeldahl |
P (mg kg‑1) |
9.0 |
Olsen |
After a relatively homogeneous mixture of soil was obtained, the soil was weighed and transferred to PVC pots (1.5 kg of soil per pot). An increase in soil electric conductivity can affect plant growth; however, most plants are not significantly affected until electrical conductivity becomes greater than 4 decisiemens (dS) m‑1 (McCutcheon and Schnoor 2003). The soil used in this research had an electrical conductivity of 2.93 dS m‑1 (Table 1). Also, soil contamination with TPHs results in a decrease in plant nitrogen (N) absorption and an increase in the C/N ratio. In the contaminated soil used in this study, the C/N ratio was relatively high (approximately 30.9), which may lead to a decrease in phytoremediation efficiency due to N deficiency in petroleum hydrocarbon metabolisms (Xu and Johnson 1997). Hence, in order to optimize the condition of soil nutrients and to study the effect of fertilization on plant growth in contaminated soil, three types of organic fertilizers were used. These fertilizers were animal fertilizer, humus, and peat fertilizer. Characteristics of the peat fertilizer were as follows: pH = 5.5, total N = 1.1%, existing phosphorus (P) = 32.7 mg kg‑1, potassium (K) = 2280 mg kg‑1, and organic C = 30.9%. The humus contained 0.41% N, 80 mg kg‑1 P, 3400 mg kg‑1 K, pH = 7.7, EC = 1.75 dS m‑1, and organic C = 16.7%. The N and P contents in the animal fertilizer were 1.62% and 136 mg kg‑1, respectively. Other properties of the animal fertilizer were: pH = 7.8, EC = 13.9 dS m‑1, K = 25400 mg kg‑1, and organic C = 23.2%. Soil composition in the pots was as follows:
Soil C: clean soil from lands surrounding pond No. 4 of the Oil Refinery of Tehran, without any kind of contamination background (control soil);
Soil E: highly contaminated soil (80%) + clean soil (20%);
Soil I: highly contaminated soil (80%) + peat fertilizer (20%);
Soil H: highly contaminated soil (80%) + humus (20%);
Soil G: highly contaminated soil (80%) + clean soil (10%) + animal fertilizer (10%).
The initial concentration of TPHs in the soil provided by the Oil Refinery of Tehran was 50 516 mg kg‑1 (more than 5% of total weight), which demonstrates a high level of soil contamination. Taking into consideration that in the various soil combinations the homogeneous mixture consisted of 80% highly contaminated soil and 20% clean soil or fertilizer, the contamination level in soil samples E, H, G and I was more than 40 000 mg kg‑1 (40 412 mg kg‑1). A control treatment (without plants), in which contamination was naturally attenuated, was also considered.
Sorghum and common flax were cultivated over a 3-mo period from mid-August to mid-November. The pots were placed outdoors under sunlight with a light/dark cycle of approximately 12/12 h. Temperature was around 22-28°C. Monitoring of plant growth was done on d 10, 20, 30, 60 and 90. The pots were watered twice a wk to maintain a constant and sufficient moisture level. Germination rate during the initial wk was determined by counting the number of grown seeds. Shoot height was also measured and monitored. After the 3-mo growth period, plants were removed from their pots and root length and shoot height were measured. For this purpose, the plants were first carefully removed from the soil and steadily washed with running water in such a way that the roots would not be damaged. Then, using a ruler, root length and shoot height were measured. In order to measure dry biomass, plants were placed in an oven at 70°C for 48 h, and then weighed.
Soil sampling from pots has not been mentioned or discussed in most articles pertaining to phytoremediation experiments. After the plants were removed from the soil and the pots were destroyed at the end of d 90 (end of pilot experiment), the soil inside each plant was spilt on a plastic bag and completely mixed in order to obtain a homogeneous mixture of plant soil. Soil samples were not taken only from the rhizosphere zone because this might have misled conclusions about remediation efficiency for bulk soil. Therefore, approximately 100-150 g of homogeneous soil was sent to the laboratory of the University of Tehran, Faculty of Environment, for analysis.
For TPHs analysis, soil samples were air dried at room temperature and passed through a 2 mm sieve. The samples were stored at 4°C prior to extraction and analysis. Ultrasonic extraction was performed using dichloromethane as a solvent. Ten cc of dichloromethane were added to about 5 g of contaminated soil and were then placed in an ultrasonic water bath for 3 min at room temperature. All of these operations were repeated three times (US EPA 1998). The extracts obtained were then concentrated to 1 mL under a gentle stream of N gas. Two µL of the sample were injected into a gas chromatograph UNICAM 610 series equipped with a flame ionization detector (FID). The column used for analysis was DB-5 and it was 30 m long, with 0.25 mm internal diam and 0.2 µm thickness of film. Injector and FID temperatures were adjusted at 280 and 340°C, respectively. Initial column temperature was adjusted at 50°C for 5 min, and then increased to 250°C with a 10°C min‑1 slope, and remained at 250°C for 40 min.
Mean and standard error (SE) values of the three replicates (n = 3) were calculated for germination and shoot height. The difference between soil treatments was tested by a one-way ANOVA. Significance level was considered at P = 0.05. If the difference was significant, Tukey multiple comparisons were carried out to determine where the differences were. All statistical analyses were performed using the software Statistical Package for Social Sciences (SPSS) 10.0 for Windows (SPSS Inc., IL, USA).
Results and discussion
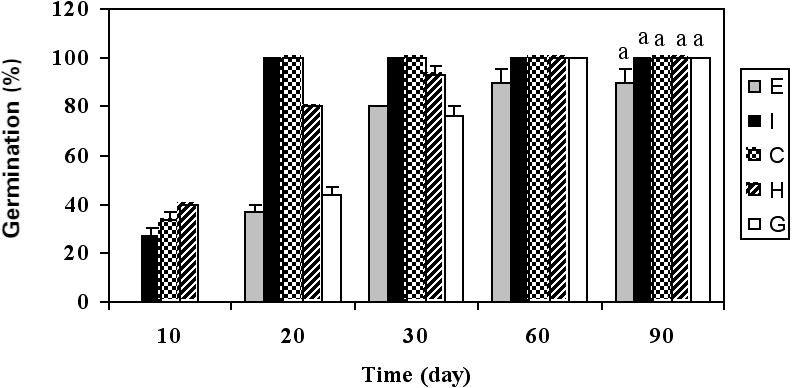
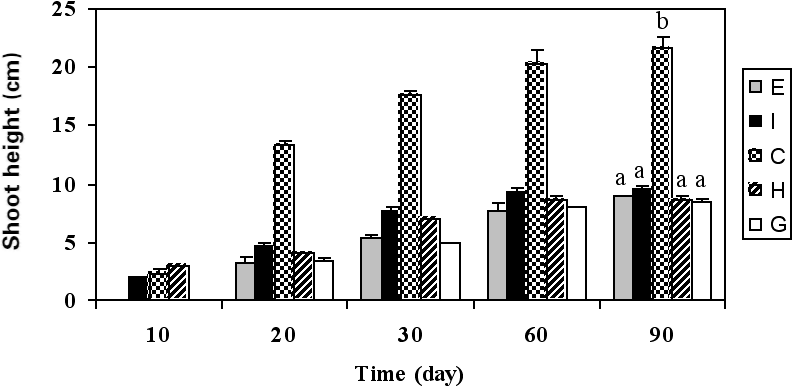
Sorghum showed a promising behaviour in highly contaminated soil. Sorghum germination was clearly visible on d 10 for treatments H and I. Germination percentage in all treatments except treatment E (90% germination) reached 100% (Fig. 1). However, treatments G and H reached their maximum germination rate later than treatments C and I. Some studies have suggested there might be a link between poor germination and subsequent poor growth in hydrocarbon-contaminated soils (Chaineau et al. 1997); however, other studies have shown the exact opposite, i.e. that germination could be unaffected but subsequent growth could still be significantly diminished (Li et al. 1997). In our study, germination of sorghum was the same with and without hydrocarbons (P < 0.05), but the subsequent growth (evaluated as shoot height after 90 d of culture) under hydrocarbon pollution conditions was significantly decreased (P < 0.05). Maximum root length was achieved in treatments C, G and I. Salanitro et al. (1997) reported a reduction in seedling emergence of corn, wheat and oat growing on soil contaminated with a heavy crude oil. Gallegos-Martinez et al. (2000) tested some native plant species of tropical Mexico in petroleum- contaminated soil and found a 30 to 90% reduction in germination. Merkl et al. (2004a) suggested that seedling emergence could be inhibited by the toxic effects of the soil or by unfavourable soil moisture conditions, especially in the Tropics. The latter reason does not apply to our greenhouse experiment because appropriate irrigation intervals were observed; however, it may be an important concern in future field experiments in Iran due to the country’s semi-arid to arid climate.
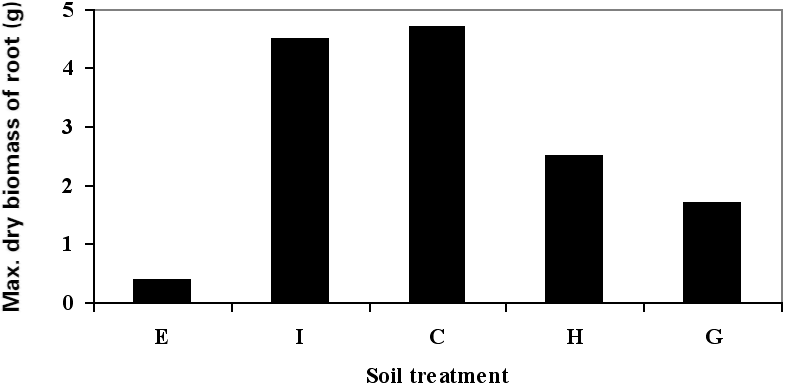
Figure 1
Growth characteristics of sorghum: (a) germination, (b) shoot height, (c) maximum root length, (d) maximum dry biomass of root, and (e) maximum dry biomass of shoot.
(a)
(b)
(c)
(d)
(e)
Among the contaminated soil treatments, the biggest value of root and shoot dry biomass was obtained with treatment I. Table 2 shows the variations in plant growth parameters under aged, petroleum hydrocarbon-contaminated soil conditions. In treatment E, in which no fertilizer was used, minimum growth was recorded. Although the use of a fertilizer may not have a considerable impact on plant tolerance or sensitivity to petroleum contamination, it may have a positive effect on plant growth, even in contaminated soils, through biostimulation. Our results indicate that the impact of peat fertilizer on sorghum growth in highly contaminated soils was more important than that of the other two types of fertilizers used in this study. On-site observations confirmed that sorghum possesses an extensive and dense root system. With regard to its root system and also to its tolerance to highly petroleum- contaminated soil, it seems that sorghum may be a promising choice for the phytoremediation of TPHs-contaminated soils.
Table 2
Range of petroleum hydrocarbons influence on changes in plant growth parameters in various soil treatments
|
Range of variation (reduction) (%) |
||||
|---|---|---|---|---|---|
Plant |
Germination |
Shoot height |
Root length |
Dry biomass of shoot |
Dry biomass of root |
Sorghum |
0 ‑ 10 |
55.8 ‑ 60.5 |
50 ‑ 76.9 |
64.2 ‑ 79.2 |
4.2 ‑ 91.5 |
Common flax |
35 ‑ 92.5 |
46.6 ‑ 63.3 |
39.1 ‑ 78.2 |
87.2 ‑ 100 |
82.7 ‑ 100 |
The growth parameters of common flax, which was used in this study for the first time in a phytoremediation experiment in Iran, were considerably decreased by oil pollution. Germination on d 10 only occurred in the control treatment and, in the other treatments (except treatment E), germination was only observed on d 20 (Fig. 2). The maximum germination rate based on surface density was first obtained with control treatment C (100%) (P < 0.05), and then with treatment I (66.7%) (P < 0.05). The use of an animal fertilizer or humus did not have a positive impact on common flax germination; moreover, germination rate in these treatments was significantly less than in unfertilized treatment E (P < 0.05). Petroleum contamination led to a considerable delay and decrease in germination among fertilized and unfertilized treatments. In a study conducted by Adam and Duncan (2002), the lack of germination of common flax seeds in soil contaminated with diesel was attributed to the penetration of hydrocarbons into the seeds, which is believed to have killed the embryos. Volatile hydrocarbons with light molecular weight are usually able to penetrate into seeds.
Figure 2
Growth characteristics of common flax: (a) germination, (b) shoot height, (c) maximum root length, (d) maximum dry biomass of root, and (e) maximum dry biomass of shoot.
(a)
(b)
(c)
(d)
(e)
The soil used in this study was aged and mainly contained high molecular weight hydrocarbons; therefore, one of the probable reasons for explaining the decrease and delay in common flax germination might be the hydrocarbons’ physical water repellent property. Hydrocarbons may act as a physical barrier around the seeds, thus preventing or reducing both water and oxygen from entering the seeds (Adam and Duncan 2002). More importantly, the poor germination observed in treatments H and G was considered to be the result not only of oil pollution and physical interference of hydrocarbons, but also of fertilizer quality and over-fertilization. Animal and humus fertilizers are not produced under standard procedures in Iran; however, they are extensively used for agricultural purposes. For instance, the electrical conductivity of the animal fertilizer was not in a favourable range for plant germination (EC > 4 dS m‑1). In addition, over-fertilization was probably responsible for the poor germination of common flax in treatments H and G, as Brandt et al. (2006) also reported its adverse effect on the germination of Vetiver (Vetiveria Zizanioides (L.) Nash) cultivated in oil-polluted soil. Applying a smaller dose of these two fertilizers might improve the results to some extent. In the case of the animal fertilizer, which is basically applied to the soil surface, lower fertilizer levels and/or delayed fertilization are suggested for future studies since seeds might have been damaged by direct exposure to the fertilizer at the first stages of the growth period; Merkl et al. (2004b) obtained the same results with different fertilizers. Control treatments for the three fertilizers used in this study were also considered. The influence of fertilizers on plant growth in contaminated soil was consistent with the results obtained with clean soil (data not shown). The humus and animal fertilizer did not show a positive effect on seed germination in clean soil.
Shoot height of common flax diminished significantly in the presence of hydrocarbons (P < 0.05). Maximum root and shoot dry biomass as well as maximum root length were obtained in the uncontaminated treatment (C). Although the use of organic fertilizers (especially peat fertilizer) affected the length and biomass of common flax organs, this effect was not considerable. Petroleum hydrocarbon contamination decreased root length and the shoot and root dry biomass of common flax by 39.1-78.2, 87.2-100 and 82.7-100%, respectively (Table 2). Considering in situ observations, root density of common flax was considerable in the various treatments. A significant reduction in plant biomass caused by the presence of petroleum hydrocarbons, as found in the present study, was also reported in the literature. Chaineau et al. (1997) reported a growth rate reduction of more than 80% in beans and wheat. Merkl et al. (2004b) also found a significant shoot length reduction in the presence of 3 and 5% crude oil. Liste and Felgentreu (2006) reported a shoot biomass reduction of 38.9% for ryegrass cultivated in contaminated soil (TPHs initial concentration of 1517 mg kg‑1) over a 95-d period. Root biomass also diminished by 52.6% in their study. Inhibition of plant growth can be caused by toxic compounds in petroleum hydrocarbons, especially low molecular weight hydrocarbons (Bossert and Bartha 1985; Merkl et al. 2004b). Dry biomass was less influenced in the present study in comparison with the other studies mentioned above, probably because of the lower contents of low molecular weight hydrocarbons in the highly weathered soil used in our study. Hydrocarbons can also alter soil properties because of their hydrophobicity, which may result in a reduction in water and nutrient availability and, therefore, in plant growth.
Sorghum showed better germination rates than common flax in contaminated soil. The germination rate in treatment G was less important than in the other treatments. After the control treatment, treatment I had the best rate of germination. On-site observations showed considerable root density in both sorghum and common flax. Among the organic fertilizers used in this study, the peat fertilizer had the best influence on growth parameters of plant species cultivated in hydrocarbon-contaminated soils. The animal fertilizer had the weakest impact. Performance of humus was average and, in comparison with the unfertilized treatment, it did not show considerable effect on most measured growth parameters; however, its effect on root dry biomass was positive. These differences may be attributed to the different compositions of the fertilizers. The N content in the animal fertilizer was relatively higher than in the humus and peat fertilizer, but the animal fertilizer’s very high EC value (13.9 dS m‑1) caused strong adverse effects on plant growth. For instance, common flax germination and biomass both decreased when using animal fertilizer in comparison with the unfertilized treatment (treatment E). The more effective influence of peat on plant growth in comparison with humus may be attributed to both a higher N content and a lower pH value. Soil pH can affect the availability of nutrients and their absorption by plant roots. Mildly acidic pH values can increase nutrient absorption. The humus fertilizer and contaminated soil had almost similar pH values (around 7.7), while the peat fertilizer had a pH value of 5.5; it therefore slightly reduced the soil pH in treatment I.
Taking into consideration the results of the previous section, treatment I, in which the plants showed the best growth characteristics, and the unfertilized treatment (E) were chosen for TPHs analysis. The quantity of hydrocarbon removed from the soil by sorghum and common flax was determined to evaluate their phytoremediation efficiency. Results are shown in Table 3. A decrease in TPHs was observed over the course of the experiment in all treatments (E, I and control). In unplanted soil, 41.66% of TPHs disappeared over the 90-d period. Despite plant growth reduction, enhanced degradation of hydrocarbons attributable to the presence of plants was detected in sorghum (treatment I) and common flax (treatments E and I). Maximum removal (45.97%) was obtained with treatment I, in which common flax removed more than 18 500 dS m‑1 of TPHs from the soil over the course of the experiment, compared with the control. The quantity of TPHs removed in treatment I was almost twice higher than that removed in unplanted soil. Sorghum reduced TPHs levels by 3.47 and 23.63% in treatments E and I, respectively, compared with the control. The observed enhanced degradation of hydrocarbons in the presence of plants is related to the increased microbial activity that is attributable to root exudates and oxygen input from roots, as Escalante-Espinosa et al. (2005) also observed. Some studies suggest that plants under stress seem particularly effective in promoting hydrocarbon biodegradation because their roots release greater amounts of certain phenolic chemicals (e.g. salicylate) that induce microbial hydrocarbon degradation (Kamath et al. 2004; Liste and Felgentreu 2006). Merkl et al. (2005) also reported a significant effect of Brachiaria brizantha (Hochst. ex A. Rich.) Stapf. and Cyperus aggregatus (Willd.) Endl. plants on total oil and grease (TOG) content in soil. In a phyoremediation study by Tischer and Hubner (2002), Phragmites australis (Car.) Trin. ex Steud., Alnus glutinosa (L.) Gaertn., and Robinia pseudoacacia L. reduced mineral oil hydrocarbon contents by more than 43, 64 and 51%, respectively, after 16 wk. In the comparison between the planted and unplanted treatments, hydrocarbon removal was more than 40% higher in the planted pots than in the unplanted pots in their experiment, which is comparable to the results obtained for treatment I with common flax but higher than the results obtained with the other planted treatments in the present study. The lower phytoremediation efficiency in our experiment may originate from the fact that the soil used was extremely weathered, and it mostly contained heavy and persistent fractions of hydrocarbons. However, hydrocarbon fraction analysis (saturates, aromatics and polars) should be, whenever possible, included in future phytoremediation studies in order to obtain more detailed information about fraction changes over the plant growth period.
Table 3
Variation in TPHs concentrations after phytoremediation
Plant treatment |
TPHs reduction1 (mg kg‑1) |
Total removal of TPHs (%) |
Concentration change2 (mg kg‑1) |
Net efficiency of phytoremediation (%) |
|---|---|---|---|---|
Sorghum ‑ E3 |
18 240 ± 2145 |
45.13 ± 0.53 |
‑1 404 ± 402 |
3.47 ± 1.0 |
Sorghum ‑ I4 |
26 387 ± 69 |
65.29 ± 1.71 |
‑9 551 ± 1175 |
23.63 ± 1.41 |
Common flax ‑ E |
31 400 ± 516 |
77.7 ± 1.28 |
‑14 564 ± 618 |
36.04 ± 1.49 |
Common flax ‑ I |
35 414 ± 517 |
87.63 ± 1.28 |
‑18 578 ± 1062 |
45.97 ± 0.76 |
Control |
16 836 ± 597 |
41.66 ± 1.48 |
- |
- |
Compared with initial concentration.
Compared with control.
E = Soil highly contaminated (80%) + clean soil (20%).
I = Soil highly contaminated (80%) + peat fertilizer (20%).
Values represent the mean ± standard deviation, n = 3.
There was a difference in the ability of sorghum and common flax to accelerate petroleum hydrocarbons removal in our study. The phytoremediation potential of common flax was higher than that of sorghum in both the fertilized and unfertilized treatments; however, common flax growth was relatively more reduced by the presence of hydrocarbons. This might have been caused by the different composition of the exudates and, therefore, by their distinct impact on microbial activity in the rhizosphere of each plant species since the growth and activity of degrading micro-organisms are stimulated by root exudates. It is widely accepted that the potential to decontaminate polluted soils differs among plant species and even among varieties (Wiltse et al. 1998). No significant remedial effect was observed for the unfertilized sorghum treatment despite its successful growth in the contaminated soil. The decreased microbial growth and metabolism caused by N deficiency in the root zone due to the plant’s uptake of large amounts of N from unfertilized soil could explain these results. Liste and Felgentreu (2006) even observed an inhibitory effect in ryegrass on the degradation of petroleum hydrocarbons. Fertilization improved the phytoremediation efficiency of sorghum significantly.
Peat fertilizer showed a positive effect on phyto- remediation efficiency in both sorghum and common flax. Since the main mechanism of phytoremediation in oil-polluted soils is based on the stimulation of soil micro-organisms, it can be assumed that the higher root biomass obtained with plants provides a larger rhizosphere for the microbial population and, therefore, an enhanced degradation of petroleum hydrocarbons in soils. Moreover, the improvement of soil nutrient conditions through peat addition can further enhance hydrocarbon biodegradation compared with the unfertilized treatment (E). Tejada et al. (2008) and Merkl et al. (2005) also observed that oil degradation could possibly be further enhanced by improving plant growth through fertilizer optimization.
The phytoremediation potential of sorghum and common flax in highly oil-polluted soils as well as the effects of TPHs on the growth parameters of these plants were evaluated in this study. Results indicate that both plant species were effective and promising for the removal of TPHs from highly contaminated aged soil. The use of fertilizers may promote plant growth even in oil-contaminated soils and thereby positively affect phytoremediation efficiency, as observed in this study. Sorghum and common flax reduced TPHs levels in contaminated soil by 23.63 and 45.97%, respectively, compared with the control. Both can be introduced as tolerant plant species in highly oil-polluted soils and as new phytoremediating plant species, especially when peat fertilizer is added. Finding new tolerant plant species and studying the rate of petroleum hydrocarbons removal by sorghum and common flax are suggested for further studies. The use of vegetation as a feasible remediation approach for soils contaminated with petroleum hydrocarbons may become attractive in both developing and developed countries because it is inexpensive and requires minimum maintenance and little management. In future investigations, organic fertilizer levels should be optimized to further increase plant growth and hydrocarbon degradation.
Appendices
Acknowledgements
This research was funded by the Oil Refinery of Tehran. The authors thank the manager and HSE authorities of the Oil Refinery of Tehran for their assistance.
References
- Adam, G., and H. Duncan. 2002. Influence of diesel fuel on seed germination. Environ. Pollut. 120 : 363-370.
- Bossert, I., and R. Bartha. 1985. Plant growth on soils with a history of oily sludge disposal. Soil Sci. 140 : 75-77.
- Brandt, R., N. Merkl, R. Schultaze-Krafl, C. Infante, and G. Broll. 2006. Potential of Vetiver (Vetiveria Zizanioides (L.) Nash) for phytoremediation of petroleum hydrocarbon-contaminated soils in Venezuela. Int. J. Phytoremediat. 8 : 273-284.
- Chaineau, D.H., J.L. Morel, and J. Oudot. 1997. Phytotoxicity and plant uptake of fuel oil hydrocarbons. J. Environ. Qual. 26 : 1478-1483.
- Chekol, T., L.R. Vough, and R.L. Chaney. 2004. Phytoremediation of polychlorinated biphenyl-contaminated soils: the rhizosphere effect. Environ. Int. 30 : 799-804.
- Cunningham, S.D., and D.W. Ow. 1996. Promises and prospects of phytoremediation. Plant Physiol. 110 : 715-719.
- Escalante-Espinosa, E., M.E. Gallegos-Martınez, E. Favela-Torres, and M. Gutierrez-Rojas. 2005. Improvement of the hydrocarbon phytoremediation rate by Cyperus laxus Lam. inoculated with a microbial consortium in a model system. Chemosphere 59 : 405-413.
- Frick, C.M., R.E. Farrell, and J.J. Germida. 1999. Assessment of Phytoremediation as an In-Situ Technique for Cleaning Oil-Contaminated Sites. Petroleum technology alliance of Canada, Calgary, Canada. 82 p.
- Gallegos-Martinez, M.G., A.G. Santos, L.G. Cruz, M.A. Garcia, L.Y. Trujillo, Z. Liz, and M. Gutierrez-Rojas. 2000. Diagnostic and resulting approaches to restore petroleum-contaminated soil in a Mexican tropical swamp. Water Sci. Technol. 42 : 377-384.
- Huang, X.D., Y. El-Alawi, D.M. Penrose, B.R. Glick, and B.M. Greenberg. 2004. A multi-process phytoremediation system for removal of polycyclic aromatic hydrocarbons from contaminated soils. Environ. Pollut. 130 : 465-476.
- Huang, X.D., Y. El-Alawi, J. Gurska, B.R. Glick, and B.M. Greenberg. 2005. A multi-process phytoremediation system for decontamination of persistent total petroleum hydrocarbons (TPHs) from soils. Microchem. J. 81 : 139-147.
- Johnson, D.L., D.R. Anderson, and S.P. McGrath. 2005. Soil microbial response during the phytoremediation of a PAH contaminated soil. Soil Biol. Biochem. 37 : 2334-2336.
- Joner, E.J., D. Hirmann, O.H.J. Szolar, D. Todorovic, C. Leyval, and A.P. Loibner. 2004. Priming effects on PAH degradation and ecotoxicity during a phytoremediation experiment. Environ. Pollut. 128 : 429-435.
- Kamath, R., J.L. Schnoor, and P.I. Alvarez. 2004. Effect of root-derived substrates on the expression of nah-lux genes in Pseudomonas fluorescens HK44: implications for PAH biodegradation in the rhizosphere. Environ. Sci. Technol. 38 : 1740-1745.
- Karthikeyan, R., and A. Bhandari. 2001. Anaerobic biotransformation of aromatic and polycyclic aromatic hydrocarbons in soil microcosms: a review. J. Hazard. Subst. Res. 3 : 1-19.
- Khan, A.G. 2005. Role of soil microbes in the rhizospheres of plants growing on trace metal contaminated soils in phytoremediation. J. Trace Elem. Med. Biol. 18 : 355-364.
- Li, X., Y. Feng, and N. Sawatsky. 1997. Importance of soil water relations in assessing the endpoint of bioremediated soils. Plant Soil 192 : 219-226.
- Liste, H. and D. Felgentreu. 2006. Crop growth, culturable bacteria, and degradation of petrol hydrocarbons (PHCs) in a long-term contaminated field soil. Appl. Soil Ecol. 31 : 43-52.
- Luepromchai, E., W. Lertthamrongsak, P. Pinphanichakarn, S. Thaniyavarn, K. Pattaragulwanit, and K. Juntongjin. 2007. Biodegradation of PAHs in petroleum-contaminated soil using tamarind leaves as microbial inoculums. Songklanakarin J. Sci. Technol. 29 : 515-527.
- McCutcheon, S.C., and J.L. Schnoor. 2003. Overview of phytotransformation and control of wastes. Pages 1-58 in S.C. McCutcheon and J.L. Schnoor (eds.), Phytoremediation: Transformation and Control of Contaminants. John Wiley & Sons, New Jersey, USA.
- Merkl, N., R. Schultze-Kraft, and C. Infante. 2004a. Phytoremediation of petroleum-contaminated soils in the tropics - Pre-selection of plant species from eastern Venezuela. J. Appl. Bot. Food Qual. 78 : 185-192.
- Merkl, N., R. Schultze-Kraft, and C. Infante. 2004b. Phytoremediation in the tropics - The effect of crude oil on the growth of tropical plants. Bioremediat. J. 8 : 177-184.
- Merkl, N., R. Schultze-Kraft, and C. Infante. 2005. Assessment of tropical grasses and legumes for phytoremediation of petroleum-contaminated soils. Water Air Soil Pollut. 165 : 195-209.
- Mueller, K.E., and J.R. Shann. 2006. PAH dissipation in spiked soil: Impacts of bioavailability, microbial activity, and trees. Chemosphere 64 : 1006-1014.
- Parrish, Z.D., M.K. Banks, and A.P. Schwab. 2005. Assessment of contaminant lability during phytoremediation of polycyclic aromatic hydrocarbon impacted soil. Environ. Pollut. 137 : 187-197.
- Salanitro, J.P., P.B. Dorn, M.H. Hueseman, K.O. Moore, I.A. Rhodes, L.M. Jackson, T.E. Vipond, M.M. Western, and H.L. Wisniewksi. 1997. Crude oil hydrocarbon bioremediation and soil ecotoxicity assessment. Environ. Sci. Technol. 31 : 1769-1776.
- Tahooni, S. 2000. Principles of Foundation Engineering. Pars Aain Press, Tehran. 934 p.
- Tejada, M., J.L. Gonzalez, M.T. Hernandez, and C. Garcia. 2008. Application of different organic amendments in a gasoline contaminated soil: Effect on soil microbial properties. Bioresour. Technol. 99 : 2872-2880.
- Tesar, M., T.G. Reichenauer, and A. Sessitsch. 2002. Bacterial rhizosphere populations of black poplar and herbal plants to be used for phytoremediation of diesel fuel. Soil Biol. Biochem. 34 : 1883-1892.
- Tischer, S., and Hubner, T. 2002. Model trials for phytoremediation of hydrocarbon-contaminated sites by the use of different plant species. Int. J. Phytoremediat. 4 : 187-203.
- US EPA. 1998. Test Methods for Evaluating Solid Waste, Physical Chemical Methods. Environmental Protection Agency, Washington, DC.
- US EPA. 2000. Introduction to phytoremediation. Environmental Protection Agency, USA. Page 5.
- Wiltse, C.C., W.L. Rooney, Z. Chen, A.P. Schwab, and M.K. Banks. 1998. Greenhouse evaluation of agronomic and crude oil-phytoremediation potential among alfalfa genotypes. J. Environ. Qual. 27 : 169-173.
- Xu, J.G., and R.L. Johnson. 1997. Nitrogen dynamics in soils with different hydrocarbon contents planted to barley and field pea. Can. J. Soil Sci. 77 : 453-458.
List of figures
Figure 1
Growth characteristics of sorghum: (a) germination, (b) shoot height, (c) maximum root length, (d) maximum dry biomass of root, and (e) maximum dry biomass of shoot.
(a)
(b)
(c)
(d)
(e)
List of tables
Table 1
Physical and chemical characteristics of the experimental soil used in the phytoremediation study
Parameter |
Value |
Analytical method |
|---|---|---|
Clay (%) |
33 |
Hydrometer measurement |
Silt (%) |
33 |
Hydrometer measurement |
Sand (%) |
20 |
Hydrometer measurement |
Gravel (%) |
14 |
Sieve |
Organic matter (%) |
6.92 |
Walkley-Black |
Organic C (%) |
4.02 |
– |
Soil pH |
7.6 |
1:1 soil/water slurry |
Electrical conductivity (dS m-1) |
2.93 |
1:2 soil/water slurry |
Total N (%) |
0.13 |
Kjeldahl |
P (mg kg‑1) |
9.0 |
Olsen |
Table 2
Range of petroleum hydrocarbons influence on changes in plant growth parameters in various soil treatments
|
Range of variation (reduction) (%) |
||||
|---|---|---|---|---|---|
Plant |
Germination |
Shoot height |
Root length |
Dry biomass of shoot |
Dry biomass of root |
Sorghum |
0 ‑ 10 |
55.8 ‑ 60.5 |
50 ‑ 76.9 |
64.2 ‑ 79.2 |
4.2 ‑ 91.5 |
Common flax |
35 ‑ 92.5 |
46.6 ‑ 63.3 |
39.1 ‑ 78.2 |
87.2 ‑ 100 |
82.7 ‑ 100 |
Table 3
Variation in TPHs concentrations after phytoremediation
Plant treatment |
TPHs reduction1 (mg kg‑1) |
Total removal of TPHs (%) |
Concentration change2 (mg kg‑1) |
Net efficiency of phytoremediation (%) |
|---|---|---|---|---|
Sorghum ‑ E3 |
18 240 ± 2145 |
45.13 ± 0.53 |
‑1 404 ± 402 |
3.47 ± 1.0 |
Sorghum ‑ I4 |
26 387 ± 69 |
65.29 ± 1.71 |
‑9 551 ± 1175 |
23.63 ± 1.41 |
Common flax ‑ E |
31 400 ± 516 |
77.7 ± 1.28 |
‑14 564 ± 618 |
36.04 ± 1.49 |
Common flax ‑ I |
35 414 ± 517 |
87.63 ± 1.28 |
‑18 578 ± 1062 |
45.97 ± 0.76 |
Control |
16 836 ± 597 |
41.66 ± 1.48 |
- |
- |
Compared with initial concentration.
Compared with control.
E = Soil highly contaminated (80%) + clean soil (20%).
I = Soil highly contaminated (80%) + peat fertilizer (20%).
Values represent the mean ± standard deviation, n = 3.