Abstracts
Abstract
The virulence of ten indigenous and two commercial isolates of entomopathogenic nematodes against the black cutworm, Agrotis ipsilon, was assessed under laboratory conditions. When comparing commercial isolates, Steinernema carpocapsae exhibited higher virulence than S. feltiae. One indigenous isolate of S. carpocapsae (6Sc) provided similar or higher virulence than the commercial isolate against black cutworm larvae. An indigenous strain of S. kraussei demonstrated low virulence in our study.
Keywords:
- Biological control,
- cutworm,
- entomopathogenic nematodes,
- golf courses,
- Steinernema carpocapsae,
- Steinernema feltiae,
- turfgrass
Résumé
La virulence de dix isolats indigènes et deux isolats commerciaux de nématodes entomopathogènes contre le vers-gris noir, Agrotis ipsilon, a été évaluée en laboratoire. Lorsqu’on compare les isolats commerciaux, Steinernema carpocapsae a démontré une virulence supérieure à S. feltiae. Un isolat indigène de S. carpocapsae (6Sc) a affiché une virulence similaire ou supérieure à la formulation commerciale contre les larves de vers-gris noir. Le nématode indigène S. kraussei a démontré une faible virulence dans notre étude.
Mots-clés :
- Gazon,
- lutte biologique,
- nématodes entomopathogènes,
- Steinernema carpocapsae,
- Steinernema feltiae,
- terrains de golf,
- vers-gris
Article body
The black cutworm (BCW), Agrotis ipsilon (Hufnagel) (Lepidoptera: Noctuidae), is a polyphagous insect pest. Its larvae feed on a wide range of plant families, causing serious damage to numerous crops such as corn, wheat, vegetables and fruits (Busching and Turpin 1977). On turfgrass, BCW is recognized as the most damaging of all cutworms, particularly on golf greens and tees (Potter 1998). In Canada, BCW damage on golf courses is mostly observed in eastern Canada, where one or two insecticide treatments are typically applied on greens every year (Simard 2006). Damage takes on the form of small dead patches that resemble ball marks on golf greens, and predatory bird regularly pull up tufts of grass, thus increasing damage on the surface (Potter 1998). With the deregistration of some chemicals and new legislation restricting pesticide use on turfgrass, alternative control methods to manage BCW are sought (Bélair et al. 2010; Held and Potter 2012).
Over the years, entomopathogenic nematodes (EPNs) in the Steinernematidae and Heterorhabditidae families have demonstrated their potential as biological control agents against several insect pests in agriculture, including BCW in turfgrass (Grewal et al. 2005; Ebssa and Koppenhöfer 2011). Recently, surveys have been conducted in turf habitats to discover new EPN species or strains with enhanced control potential (Ansari et al. 2007; Redmond and Potter 2010). In eastern Canada, new indigenous isolates of EPNs have been collected on golf courses and have shown variable virulence against the European crane fly, Tipula paludosa (Meigen) (Simard et al. 2006, 2007). The objectives of this study were to assess the virulence of indigenous EPNs against BCW larvae under laboratory conditions and to evaluate the efficacy of Steinernema carpocapsae (Weiser) against BCW under greenhouse conditions.
Black cutworms used in this study originated from a population infesting a golf course green (Montebello, QC, Canada, 45°39'N, 74°57'W). The BCW colony was maintained in an environmental chamber following the method described in Reese et al. (1972). All experiments were conducted on fifth-instar larvae. Canadian EPN isolates were collected in the provinces of Quebec and Ontario and included four S. feltiae (Filipjev) [1Sf (27), 2Sf (37), 3Sf (13), 4Sf (18)], five S. carpocapsae [5Sc (34), 6Sc (34), 7Sc (28), 8Sc (27), 9Sc (8)], and one S. kraussei (Steiner) [10Sk (3)]; numbers in parentheses correspond to golf courses described in Simard et al. (2007). Prior to performing each assay, indigenous EPN isolates and commercial formulations of S. feltiae (CSf; Plant Products Co. Ltd., Laval, QC) and S. carpocapsae (CSc; Natural Insect Control Inc., Stevensville, ON) were reproduced once on Galleria mellonella L. larvae (Dutky et al. 1964). Emerging infective juveniles (IJs) were collected and stored in tap water at 6°C for 2 wk before use. For all experiments, the virulence of each EPN isolate was confirmed on 15 G. mellonella larvae before use. In all cases, mortality rate ranged between 90 and 100% at a concentration of 25 IJs/larva.
BCW larvae were placed at the centre of 9-cm-diam petri dishes whose bottom and top contained two 10-cm-diam Whatman No. 2 filter papers. Petri dishes were prepared as follows: 0.5 mL of distilled water on each filter paper, 1 mL of EPNs + distilled water on each filter paper, and 2 g of creeping bentgrass clippings (Agrostis palustris Huds. ‘Alpha’) (Gloco, Anjou, QC). Controls consisted of 1.5 mL of distilled water on each filter paper. Petri dishes were placed randomly in an environmental chamber at 24 ± 1°C, 16L:8D, for 72 h. Mortality was recorded 48 and 72 h after treatment application.
The commercial formulations of S. feltiae and S. carpocapsae were tested against BCW at the following concentrations: 0, 50, 250, 500 and 1000 IJs/larva/petri dish. For each species and concentration, the experimental unit was 10 larvae per dish with three replications (N = 3 x 10), and the experiment was repeated once for a total of 60 larvae per species per concentration. A factorial analysis of variance (ANOVA; 2 nematode species x 5 concentrations x 2 tests) followed by a protected LSD test were used to compare insect mortality between treatments (α = 0.05) (SAS Institute Inc. 1999). The effects of nematode species (48 h: F = 103.40, df = 1, P < 0.0001; 72 h: F = 70.44, df = 1, P < 0.0001) and concentrations (48 h: F = 53.20, df = 4, P < 0.0001; 72 h: F = 89.37, df = 4, P < 0.0001) were significant, but the data were pooled since no significant difference was found between tests (48 h: F = 1.86, df = 1, P = 0.1806; 72 h: F = 0, df = 1, P = 1.0). The data were normally distributed and not transformed.
In another test, the virulence of ten indigenous EPN isolates against BCW was compared with the commercial formulations of S. feltiae and S. carpocapsae at the following concentrations: 0, 50 and 250 IJs/larva/petri dish. For each isolate and concentration, the experimental unit was ten larvae per dish with five replications (N = 5 x 10). A factorial analysis of variance (ANOVA; 2 concentrations x 13 isolates) followed by a protected LSD test were used to compare insect mortality between treatments (α = 0.05) (SAS Institute Inc. 1999). Both effects, i.e. concentration (48 h: F = 127.38, df = 1, P < 0.0001; 72 h: F = 158.53, df = 1, P < 0.0001) and isolates (48 h: F = 34.27, df = 12, P < 0.0001; 72 h: F = 35.54, df = 12, P < 0.0001), were significant. The data were normally distributed and not transformed.
For the greenhouse tests, 1 mo before nematode inoculation, creeping bentgrass was seeded in plastic pots (11 cm diam, 9.5 cm high) filled with a 80% sand:20% peat (v:v) soil mixture. Turfgrass was trimmed to a 25-mm height 3 d prior to nematode inoculation, and one BCW larva was introduced per pot. The commercial formulation of S. carpocapsae was suspended in water and applied using spray bottles calibrated to deliver 50, 250, 500 and 1000 IJs/pot in a standard 3-ml volume per pot. An additional 3 mL of water was applied to drench the nematodes into the soil. For each concentration, the experimental unit was ten larvae with four replications (N = 4 x 10). The experiment was repeated once with three replications (N = 3 x 10). Insect mortality was recorded 5 d post-application. Data were analyzed together since no significant difference was observed between experiments (F = 0.30, df = 1, P = 0.5890).
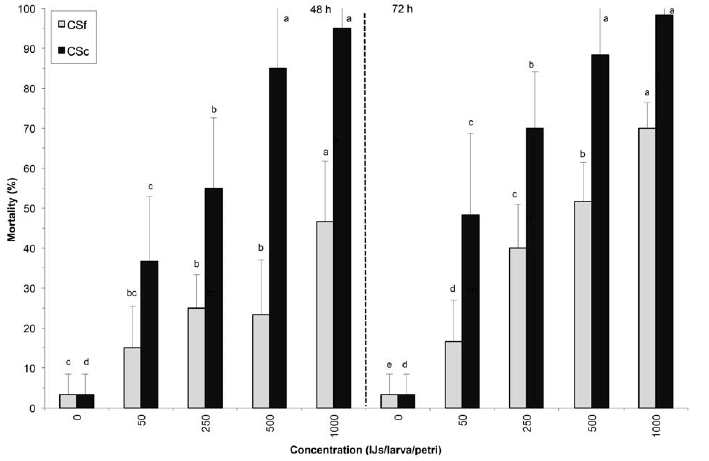
In the Petri dish tests, both commercial formulations of S. carpopcasae and S. feltiae caused significant mortality in fifth-instar larvae of BCW (Fig. 1). Steinernema carpocapsae showed higher virulence than S. feltiae, the two formulations causing 98% and 70% mortality, respectively, at the 1000 IJs concentration after 72 h. No significant increase in mortality was found between concentrations of S. carpocapsae at 500 and 1000 IJs/larva/petri dish.
Figure 1
Mortality (±SD) of Agrotis ipsilon larvae exposed to commercial formulations of Steinernema carpocapsae (CSc) and S. feltiae (CSf).
Within the same species and evaluation time, nematode concentrations followed by the same letter are not significantly different (protected LSD test; p < 0.05).
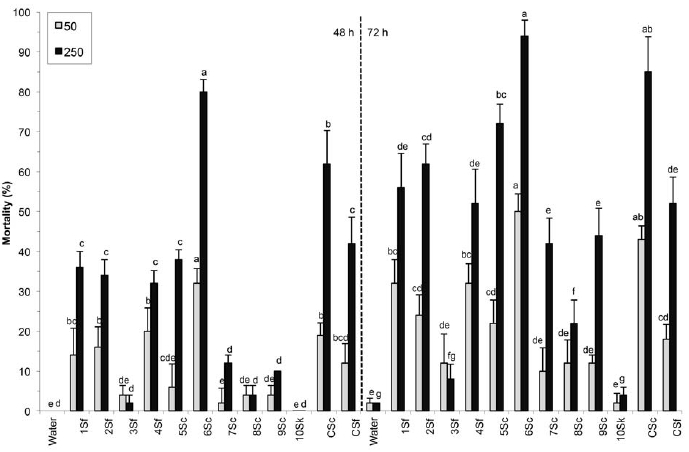
When the indigenous isolates were included, significant differences were observed among EPN isolates after 48 h (50: F = 9.19, df = 12, P < 0.0001; 250: F = 26.37, df = 12, P < 0.0001) and 72 h (50: F = 8.51, df = 12, P < 0.0001; 250: F = 32.87, df = 12, P < 0.0001) for both concentrations tested (Fig. 2). After 48 h, the highest mortality (80%) was obtained with isolate 6Sc at 250 IJs/larva/petri dish. At the low concentration, isolate 6Sc was also the most virulent with 32% mortality. After 72 h, isolates 6Sc (94%) and CSc (85%) caused the highest mortality. Virulence against BCW was similar for Canadian S. feltiae isolates and the commercial formulation. The virulence of the S. carpocapsae isolate 6Sc (higher than the commercial formulation at the low concentration) is highly desirable for a biological control agent and its potential should be investigated further under natural soil conditions.
Figure 2
Mortality (±SE) of Agrotis ipsilon larvae caused by indigenous EPN isolates (Steinernema feltiae: 1Sf, 2Sf, 3Sf, 4Sf; S. carpocapsae: 5Sc, 6Sc, 7Sc, 8Sc, 9Sc, and S. kraussei: 10Sk) compared to the S. carpocapsae (CSc) and S. feltiae (CSf) commercial formulations at 50 and 250 IJs/larva.
Within the same concentration and evaluation time, nematode isolates followed by the same letter are not significantly different (protected LSD test; p < 0.05).
Steinernema carpocapsae (commercial formulation) was efficient against BCW larvae in greenhouse trials with mortality rates higher than 50% recorded for all concentrations 5 d post-exposure (F = 14.75; df = 4; P < 0.0001). Average BCW mortality (%) for each S. carpocapsae concentration was 11%, 53%, 66%, 76% and 88% at 0, 50, 250, 500 and 1000 IJs/larva/pot, respectively. BCW mortality at the highest concentration of 1000 IJs/larva/pot was not significantly different from that recorded at 500 IJs/larva/pot.
Bioassays performed in the laboratory and greenhouse demonstrated the susceptibility of fifth-instar BCW larvae to EPNs, particularly to the species S. carpocapsae. The virulence of S. feltiae was consistently lower than that of S. carpocapsae isolates. These results are directly in line with results published previously on the susceptibility of BCW larvae to EPNs (Baur et al. 1997; Ebssa and Koppenhöfer 2011). None of the Canadian EPN isolates outperformed the two commercial formulations tested in controlling BCW. One indigenous S. carpocapsae isolate (6Sc) performed as well as the commercial formulation. The S. kraussei isolate (originally described on a sawfly host by Mráček 1977) did not exhibit any significant virulence to BCW larvae.
In the turf and lawn industry, EPNs are usually applied at a rate of 250,000 IJs m-2 (Grewal et al. 2005). Based on field experiments for BCW control in turfgrass in New Jersey, USA (Ebssa and Koppenhöfer 2011), S. carpocapsae applied at this rate provided a high and consistent control of BCW larvae on turfgrass. Under Quebec’s weather conditions, we believe that S. carpocapsae could be a useful biological tool to control BCW on golf course greens where damage is recurrent. These habitats are quite suitable for the use of EPN applications for the following reasons: 1) low turfgrass mowing height facilitates contact with BCW larvae; 2) irrigation is readily available on golf greens to prevent EPN desiccation; 3) EPN application could be made at the end of the day to minimize EPN exposure to ultraviolet light and improve contact with BCW larvae, which move on the turfgrass surface during the night; 4) adequate application equipment is available and employees are well trained. However, the high standards required on golf course greens could limit nematode application to lower maintenance sites and/or those where pesticide restrictions are implemented.
Appendices
Acknowledgements
The authors thank Nathalie Dauphinais, Yvon Fournier, Marie-Eve Gosselin, and Nicolas Turgeon for their dedicated technical assistance. This research was conducted through a collaborative research agreement between the Canadian Golf Course Superintendents Association, the Coalition for Responsible Golf, the Canadian Turfgrass Research Foundation, and Agriculture and Agri-Food Canada’s Matching Investment Initiative program.
References
- Ansari, M.A., L. Waeyenberge, and M. Moens. 2007. Natural occurrence of Steinernema carpocapsae, Weiser, 1955 (Rhabditida: Steinernematidae) in Belgian turf and its virulence to Spodoptera exigua (Lepidoptera: Noctuidae). Russ. J. Nematol. 15 : 21-24.
- Baur, M.E., H.K. Kaya, and B.E. Tabashnik. 1997. Efficacy of a dehydrated steinernematid nematode against black cutworm (Lepidoptera: Noctuidae) and diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 90 : 1200-1206.
- Bélair, G., A.M. Koppenhöfer, J. Dionne, and L. Simard. 2010. Current and potential use of pathogens in the management of turfgrass insects as affected by new pesticide regulations in North America. Int. J. Pest Manag. 56 : 51-60.
- Busching, M.K., and F.T. Turpin. 1977. Survival and development of black cutworm (Agrotis ipsilon) larvae on various species of crop plants and weeds. Environ. Entomol. 6 : 63-65.
- Dutky, S.R., J.V. Thompson, and G.E. Cantwell. 1964. A technique for the mass propagation of the DD-136 nematode. J. Insect Pathol. 6 : 417-422.
- Ebssa, L., and A.M. Koppenhöfer. 2011. Efficacy and persistence of entomopathogenic nematodes for black cutworm control in turfgrass. Biocontrol Sci. Technol. 21 : 779-796.
- Grewal, P.S., A.M. Koppenhöfer, and H.Y. Choo. 2005. Lawn, turfgrass and pasture applications. Pages 115-146 in Nematodes as Biocontrol Agents. P.S. Grewal, R.-U. Ehlers, and D.I. Shapiro-Ilan (eds.), CABI Publishing, Wallingford, UK.
- Held, D.W., and D.A. Potter. 2012. Prospects for managing turfgrass pests with reduced chemical inputs. Annu. Rev. Entomol. 57 : 329-354.
- Mráček, Z. 1977.Steinernema kraussei, a parasite of the body cavity of the sawfly, Cephaleia abietis, in Czechoslovakia. J. Invertebr. Pathol. 30 : 87-94.
- Potter, D.A. 1998. Destructive turfgrass insects: biology, diagnosis and control. Ann Arbor Press, Ann Arbor, MI, USA.
- Redmond, C.T., and D.A. Potter. 2010. Incidence of turf-damaging white grubs (Coleoptera: Scarabaeidae) and associated pathogens and parasitoids on Kentucky golf courses. Environ. Entomol. 39 : 1838-1847.
- Reese, J.C., L.M. English, T.R. Yonke, and M.L. Fairchild. 1972. A method for rearing black cutworms. J. Econ. Entomol. 65 : 1047-1050.
- SAS Institute Inc. 1999. SAS Systems for Windows, version 8. SAS Institute Inc., Cary, NC, USA.
- Simard, L. 2006. Distribution, abondance et écologie saisonnière des principaux insectes ravageurs du gazon sur les terrains de golf du Québec et évaluation du potentiel de contrôle des nématodes entomopathogènes indigènes. Thèse de doctorat, Université Laval, Québec, QC.
- Simard, L., G. Bélair, M.-E. Gosselin, and J. Dionne. 2006. Virulence of entomopathogenic nematodes (Rhabditida: Steinernematidae, Heterorhabditidae) against Tipula paludosa (Diptera: Tipulidae), a turfgrass pest on golf courses. Biocontrol Sci. Technol. 16 : 789-801.
- Simard, L., G. Bélair, S.P. Stock, and J. Dionne. 2007. Natural occurrence of entomopathogenic nematodes (Rhabditida: Steinernematidae) on golf courses in eastern Canada. Nematology 9 : 325-332.
List of figures
Figure 1
Mortality (±SD) of Agrotis ipsilon larvae exposed to commercial formulations of Steinernema carpocapsae (CSc) and S. feltiae (CSf).
Figure 2
Mortality (±SE) of Agrotis ipsilon larvae caused by indigenous EPN isolates (Steinernema feltiae: 1Sf, 2Sf, 3Sf, 4Sf; S. carpocapsae: 5Sc, 6Sc, 7Sc, 8Sc, 9Sc, and S. kraussei: 10Sk) compared to the S. carpocapsae (CSc) and S. feltiae (CSf) commercial formulations at 50 and 250 IJs/larva.