Abstracts
Abstract
The codling moth granulovirus (CpGV) and the egg parasitoids of the genus Trichogramma spp. are known as biocontrol agents of codling moth but interactions between them are unknown. The aim of this study was to assess the compatibility and the complementarity of Trichogramma minutum and CpGV. Bioassays were conducted in the laboratory the first year, as well as in an orchard over two years with the two biocontrol agents used alone or in combination. Parasitism of codling moth decreased in glass tube bioassays when CpGV was applied on eggs prior to parasitism, suggesting that female parasitoids discriminated between eggs covered or not with the virus. In contrast, egg discrimination was not observed in the orchard, since the presence of CpGV on codling moth eggs did not affect T. minutum parasitism. Both biocontrol agents together caused 77 and 79% mortality to codling moth in the orchard in the first and the second year of the study, respectively. Our results in the orchard showed that the combined use of CpGV and T. minutum to control the codling moth is compatible. Therefore, the utilization of both biocontrol agents is a promising alternative to control this pest in apple orchards.
Keywords:
- Cydia pomonella,
- Trichogramma minutum,
- CpGV,
- apple orchards,
- egg parasitoid,
- biological control,
- insect pest management
Résumé
Le granulovirus du carpocapse de la pomme (CpGV) et les parasitoïdes du genre Trichogramma sont des agents de lutte biologique du carpocapse de la pomme, mais leurs interactions sont inconnues. L’objectif de l’étude a été d’évaluer la compatibilité et la complémentarité de Trichogramma minutum et du CpGV. Des essais en laboratoire ont été menés la première année et des essais dans le verger ont été réalisés pendant deux ans avec les deux agents de lutte utilisés seuls ou en combinaison. Dans le laboratoire, le parasitisme a été réduit après que le CpGV a été appliqué sur les oeufs du carpocapse de la pomme, suggérant que les femelles parasitoïdes ont discriminé entre des oeufs recouverts de CpGV ou non. Au contraire, le CpGV n’a pas affecté le parasitisme causé par T. minutum dans le verger. Les deux agents de lutte ensemble ont causé 77 et 79 % de mortalité au carpocapse de la pomme dans le verger la première et la deuxième année respectivement. Nos résultats en verger montrent que l’effet combiné du CpGV et de T. minutum sur le carpocapse de la pomme est compatible. En conséquence, l’utilisation des deux agents de lutte biologique est une alternative prometteuse pour la lutte contre le carpocapse de la pomme dans les vergers de pommiers.
Mots-clés :
- Cydia pomonella,
- Trichogramma minutum,
- CpGV,
- vergers de pommiers,
- parasitoïde oophage,
- lutte biologique,
- lutte intégrée
Article body
INTRODUCTION
Codling moth, Cydia pomonella (L.) (Lepidoptera: Tortricidae) is a major pest in apple orchards worldwide (Barnes 1991; Witzgall et al. 2008). In North America, it is considered a primary pest, which can cause important losses to apple growers. In Quebec, Canada, the average damage at harvest in a pesticide-free plot is 22% (Chouinard et al. 2016).
Currently, mating disruption is being used to control the codling moth over 200 000 ha worldwide (Miller and Gut 2015; Morin et al. 2017). For optimal results at high pest densities, mating disruption must be assisted with supple-mentary control measures, mostly insecticide applications. Currently, codling moth granulovirus (CpGV) is being used as a bioinsecticide, in integrated fruit production programs, as an additional application to control codling moth. CpGV, which is indigenous to North America, is highly specific to codling moth larvae (Arthurs and Lacey 2004) and the oriental fruit moth, Grapholitamolesta Busck, a related species (Lacey et al. 2005). Arthurs et al. (2005) that CpGV could reduce the proportion of deep entries of codling moth larvae on apples by 77-98%. Nevertheless, CpGV is not always as effective because solar radiation deteriorates viral bodies within days (Ballard et al. 2000a; Lacey et al. 2004; Wu et al. 2015). The virus has also a short opportunity to infect codling moth, as 1st instar larvae enter fruits quickly after they hatch and are often out of reach (Jaques et al. 1994). Thus, larvae must ingest a lethal dose of CpGV between the moment they hatch and the moment they enter a fruit (Lacey et al. 2008). LD50 has been estimated to less than five occlusion bodies per neonate larvae per ml (Lacey et al. 2008). Repeated applications of CpGV products can further the development of resistant codling moth populations (Schmitt et al. 2013). To avoid this problem, the combination of a complementary biocontrol agent could enhance codling moth control, reduce the number of required CpGV applications and delay the onset of resistance.
Egg parasitoids of the genus Trichogramma have shown encouraging results in controlling codling moth (Aubry 2008; Blomefield and Giliomee 2012; Botto and Glaz 2010; Cossentine and Jensen 2000; Kienzle et al. 2012; Lacey and Unruh 2005; Sigsgaard et al. 2017). In South Africa, codling moth eggs mortality increased by 13% from spring to summer due to the egg parasitoid T. luteum Girault (Blomefield and Giliomee 2012). In Californian apple orchards, T. platneri Nagarkatti releases decreased codling moth damage by 60% (Mills et al. 2000). Additionally, females of Trichogramma can differentiate between suitable and inadequate hosts (Lobdell et al. 2005; Monje et al. 1999; Zhang et al. 2014), which makes this parasitoid a good candidate to be used in combination with other control methods.
Both, codling moth eggs and larvae occur simultaneously in southeastern Canada’s orchards during a large part of the season (Cormier et al. 2015). Since Trichogramma spp. target eggs and CpGV infects larvae of codling moth, the combined utilization of both biocontrol agents could increase the efficiency of C. pomonella control. However, using two biocontrol agents together can be difficult if antagonistic interactions occur (Denoth et al. 2002; Gonzalez et al. 2016; Snyder and Ives 2003). In a review of over 170 projects, involving the introduction of multiple biocontrol agents, Denoth et al. (2002) concluded that negative interactions between agents may play a significant role in biocontrol efficiency. For example, the larval parasitoid Trybliographa rapae Westwood (Hymenoptera: Figitidae) is susceptible to Beauveria bassiana (Balsamo) (Hypocreales: Clavicipitaceae) (Rännbäck et al. 2015), an entomopathogen that infects a variety of insects, such as aphids, thrips, and whiteflies (Zimmermann 2007). Trybliographa rapae females lay fewer eggs in healthy host larvae compared to infected larvae by B. bassiana (Rännbäck et al. 2015). Furlong and Pell (2000), also found an incompatibility between the fungal entomo-pathogen Zoophtora radicans (Brefeld) (Entomophthorales: Entomophthoraceae) and two larval parasitoids of the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae). In this case, immature parasitoids died in fungi-infested hosts.
Conversely, additive or synergistic effects between agents can increase their total efficacy and improve pest control. This is the case of entomopathogenic nematodes (EPN), used in combination with Bacillus thuringiensis subspecies japonensis (Bacillales: Bacillaceae), which demonstrated to offer a potent tool to control white grubs (Coleoptera: Scarabaeidae) (Koppenhöfer and Kaya 1997). Likewise, the combined used of the fungus Metarhizium anisopliae (Metsch) (Hypocreales: Clavicipitaceae) and the EPN Steinernema kraussei (Rhabditida: Steinernematidae) caused 100% mortality to black vine weevil larvae, Otiorhynchus sulcatus (Fabricius) (Coleoptera: Curculionidae) in British strawberries (Ansari et al. 2010). In Denmark organic orchards, where mating disruption is used to control the codling moth, three Trichogramma species that were evaluated at the same time demonstrated to be a promising alternative to control the pest (Sigsgaard et al. 2017).
Trichogramma minutum Riley is the most indigenous and abundant egg parasitoid found in Canadian apple orchards and it has shown great potential to parasitize C. pomonella eggs in Quebec (Aubry 2008; Cormier et al. 2017; Yu et al. 1984). Considering the lack of information about interactions between CpGV and T. minutum, the aim of the present investigation was to assess the simultaneous utilization of both biocontrol agents against codling moth. We hypothesize that both, CpGV and T. minutum are compatible and their combined use will have at least an additive effect controlling codling moth because female parasitoids will be able to discriminate between codling moth eggs covered and non-covered by the virus.
MATERIALS AND METHODS
Cydia pomonella (L.)
Codling moth adults were obtained from Okanagan-Kootenay Sterile Insect Release Program (OKSIR) rearing facilities in Osoyoos, British Columbia, Canada. Adults were sent by plane in Petri dishes to the Laboratoire de lutte biologique of Université du Québec à Montréal, where they were placed in rearing chambers at 23 °C, 60% RH and 16L:8D. They were kept in 2-L plastic bags filled with air in groups of approximately 50 adults and were fed a mix of distilled water and honey (50:50). Plastic bags holding eggs no older than 24 h were cut into pieces (2 cm × 2 cm). These plastic pieces containing eggs were placed in a refrigerator at 3 °C for a maximum of 4 days until used in the laboratory bioassays or orchard trials (Moffitt and Burditt 1989).
Cydia pomonella granulovirus, CpGV
The first year, a commercial formulation of CpGV (Madex®: Andermatt Biocontrol®, Grossdietwil, Switzerland) was used in laboratory bioassays and in orchard trials. The recommended rate of 100 mL ha-1 at a concentration of 3 × 1013 viral granules L-1 was applied to apple trees. Sugar was added in the formulation as a phagostimulant at a concentration of 0.5% m v-1 since it has been shown that sucrose function as an attractant and/or feeding stimulant for codling moth larvae (Ballard et al. 2000a). Similarly, milk increase CpGV persistence (Lacey et al. 2008) acting as a UV protector. Thus, in this study, powdered milk was added to Madex® at a concentration of 0.35% m v-1. In field studies, the viral suspension was applied on foliage until runoff, using a backpack sprayer.
The second year, as we were unable to obtain a federal permit to use Madex®, due to registration issues, the commercially available formulation Virosoft CP4® (Biotepp Inc., Mont-Saint-Hilaire, Quebec, Canada) was used in field studies at the recommended rate (250 mL ha-1 at a concentration of 4 × 1013 viral granules L-1). The suspension was applied with a backpack sprayer. No additives were added to the formulation, as recommended by the manufacturer.
Both, Madex® and Virosoft CP4® were applied after 6:00 p.m. to minimize deterioration of virus particles by UV radiations. All commercial CpGV products before 2000 were based on the Mexican isolate CpGV-M (Lacey et al. 2008), as is the case of Madex. Virosoft CP4® was isolated from codling moths collected in several regions in Quebec, Canada (Vincent et al. 2007).
Trichogramma minutum Riley
Trichogramma wasps used for mass releases were reared in facilities at the Institut de recherche et de développement en agroenvironnement (IRDA) in Saint-Hyacinthe, Quebec, Canada. The strain used was recovered from obliquebanded leafroller, Choristoneura rosaceana (Harr.) (Lepidoptera: Tortricidae) eggs collected in 2002, from a nearby apple orchard. Adult parasitoids were fed a mix of distilled water and honey (50:50) and were reared at 23 °C, 60% RH and 16L:8D in ventilated glass tubes.
For releases in the orchard, trichograms were multiplied in rearing cages. Mediterranean flour moth Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) eggs were offered to females and parasitized eggs were glued on Para-bio® trichocards (Saint-Augustin-de-Desmaures, Quebec, Canada) with non-toxic glue for field releases, Mucilage Ross® Craft (Ross Products, Toronto, Ontario, Canada). The number of eggs on trichocards was estimated by randomly selecting three sections per card in three cards and counting the number of eggs on 1 cm2 on each section. Then, these numbers were extrapolated to the full size of the trichocard. Each trichocard contained approximately 4 000 E. kuehniella eggs, of which some 60% were parasitized by T. minutum. Counts of parasitized eggs were carried out weekly to assess parasitism rate. Parasitoids were synchronized (they were kept in a cold chamber until placement in the orchard) to emerge one day after trichocards were placed in the orchard.
Laboratory bioassays: compatibility of biocontrol agents
Laboratory bioassays were performed during the first year of the study. Pieces of plastic bags with 10 freshly laid codling moth eggs were cut and glued on 8-cm long pieces of paper. CpGV was applied on the eggs with a hand sprayer at three different concentrations: 0.01X, 1X (recommended label concentration – 3 × 1013 viral granules L-1), and 100X. Control consisted of distilled water. Additionally, a treatment of sugar (0.5% m v-1) and milk (0.35% m v-1) was included to assess the effect of both on parasitism, since they were added to the Madex® formulation used in the orchard trials. Consequently, five treatments were compared: control; milk and sugar solution; CpGV 0.01X; CpGV 1X; and CpGV 100X.
Eggs were then allowed to dry in the laboratory and each piece of paper containing 10 eggs was placed in a 10-cm long bioassay tube. Three T. minutum females, not older than 24 h, were then introduced in each tube and left for 48 h in an environmental chamber at 23 °C, 60% RH and 16L:8D. Next, females were removed, and bioassay tubes were returned to the environmental chamber. Parasitized eggs turned black after 3 to 4 days and parasitism was assessed after 5 days by counting the black eggs. Fifteen replicates were considered in each treatment.
Field study site
Field experiments were performed at IRDA’s experimental orchard in Saint-Bruno-de-Montarville, Quebec, Canada (45°31’38’’N, 73°20’31’’W), in 2006 and 2008. The distance between trees was 1.25 m and the distance between rows was 3.65 m. Each experimental unit consisted of eight rows of 15 apple trees. Treatments and sampling were performed in the two central rows of each experimental unit. Treatments were distributed in a randomized complete block design, with four blocks. Blocks were allocated to either McIntosh or Empire cultivars. Six apple rows acted as a buffer between the sampling areas. No insecticides, acaricides or fungicides were applied in experimental plots for the duration of the study.
In all experimental units, incidence of parasitism was assessed by using codling moth sentinel eggs. Sentinel egg sets consisted in 10 to 15 codling moth eggs laid on a piece of plastic bag and stapled on the upper surface of apple leaves. One set of sentinel eggs was placed on each of 10 trees in the experimental unit at a height of approximately 1.50 m. In the second year, we used more eggs to compensate for observed losses by rain and wind in the first year. Each egg set contained 25 to 50 codling moth eggs. One set of sentinel eggs was placed at a height of approximately 1.50 m every three trees in two rows of 15 trees, for a total of 10 trees with sentinel eggs in each experimental unit. Sentinel eggs were covered with CpGV at the recommended rate, prior to parasitism. After being exposed to parasitism for four days in the orchard, sentinel eggs were brought to the laboratory, placed in Petri dishes, and incubated at 23 °C, 60% RH and 16L:8D. Five days after incubation, sentinel eggs were examined under a binocular magnifier to assess incidence of parasitism, calculated as the number of parasitized sentinel egg sets containing at least one parasitized egg on the total number of sentinel egg sets. Sentinel eggs were then kept in the incubator and the number of emerging codling moth larvae was monitored. Neonate larvae emerging from sentinel eggs were individually placed in a 2-cm long glass bioassay tube with fresh uncontaminated artificial diet, and then returned to the environmental chamber. Codling moth larval mortality was evaluated after 10 days and confirmed by gently hitting the larvae with a soft paintbrush. Dead larvae infected by CpGV liquified when touched by the paintbrush.
Egg mortality due to parasitism was added to larval mortality to express the combined codling moth mortality attributable to both biocontrol agents. Combined mortality was calculated for each treatment in summer (both years).
Four treatments were compared in the experimental orchard in the first and second years:
Control: no chemical insecticides or biocontrol agents applied. In the first year, the control consisted of sugar, powdered milk, and water. In the second year, only water was applied;
CpGV: consisted in one treatment of CpGV (Madex® in the first year and Virosoft CP4® in the second year) at the recommended label rate;
Trichogramma (TM): consisted in one mass release of T. minutum at an expected rate of 0.8 million parasitoids ha-1;
CpGV + TM: the combination of T. minutum and virus treatments at the same rates as above.
In treatments with CpGV + TM, same sequence as in the laboratory bioassays was performed by first, applying CpGV on sentinel eggs and then releasing T. minutum for egg parasitism. For mass release of T. minutum (TM and CpGV + TM), there were four release points per experimental unit. Two trichocards were placed on each of the two rows: one in the upper-middle part of the row and the other on the lower-middle part of the row. T. minutum adults were synchronized to emerge from trichocards one day after they were placed on trees. The experiment was repeated twice in the first year (on the first and last weeks of August) and twice in the second year (in mid and late July).
Statistical analyses
Analyses of variances followed by Student t-test were used for most analyses after data were angular transformed to meet normality assumption. To compare the observed combined mortality of CpGV and T. minutum with the expected combined mortality, a one-way ANOVA was used (Sokal and Rohlf 1995). When a normal distribution of data sets could not be achieved by transformations, the nonparametric Wilcoxon and Steel-Dwass all pairs tests (α = 0.05) (SAS Institute 2018) were used. All analyses were conducted using JMP software v.14.0.0 (SAS Institute, Cary, NC).
RESULTS
Laboratory bioassays: compatibility of biocontrol agents
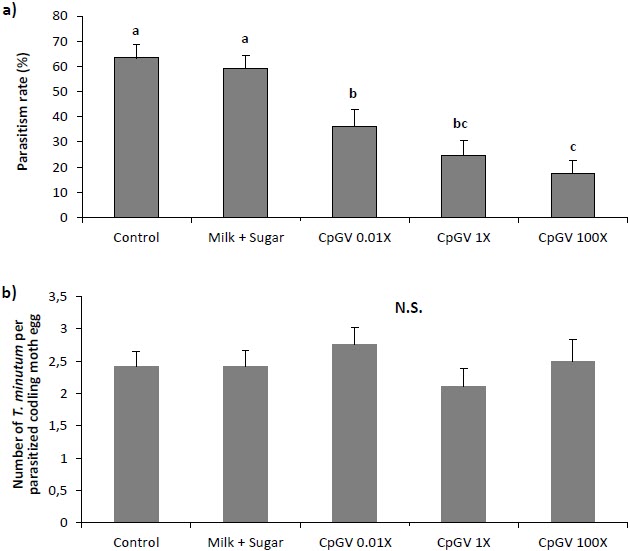
Presence of CpGV on the surface of codling moth eggs reduced parasitism rate by T. minutum females in the laboratory. Parasitism rate decreased as the concentration of the viral formulation increased (from 36.2% in the CpGV 0.01X to 17.5% in the CpGV 100X) (Fig. 1a). There was a significant difference between the control and all CpGV concentration treatments (F(4,70) = 12.2, P < 0.0001). Moreover, parasitism rate was 3.7 times higher in the control than in CpGV 100X. Parasitism rate at the recommended dose of CpGV was 24.6%. Additionally, milk and sugar did not affect parasitism rate compared to the control.
When comparing the number of emerging T. minutum per parasitized egg, no differences were found among treatments (F(4,70) = 0.705, P = 0.592) (Fig. 1b). An average of 2.5 parasitoids emerged from parasitized egg in CpGV treatments. There were no significant differences in sex ratios between treatments.
Figure 1
Effect of CpGV on parasitism of codling moth eggs by T. minutum in the laboratory. a) Rate of parasitized eggs ± SE. b) Average number of T. minutum offspring emerged per parasitized codling moth egg ± SE (ANOVA, P < 0.05)
Orchard trials
Incidence of parasitism: first year
There was a significant difference in the incidence of parasitism between the control and the CpGV + TM treatment, but there was no significant difference among the control, TM (40%), and CpGV (26.5%) treatments (F(3,12) = 5.45, P = 0.044) (Fig. 2). Incidence was 3 to 3.5 times higher in the combined treatment (63.8%) than in the control (18.3%).
Incidence of parasitism: second year
Incidence of parasitism of codling moth sentinel eggs was low in all treatments. The highest parasitism rate observed in the CpGV + TM treatment (19.8%) was significantly higher than the lowest parasitism rate observed in the CpGV treatment (9.2%) (X23 = 46.52, P = 0.0423). Incidence of parasitism was similar between the control and the TM treatment (Fig 2).
Figure 2
Incidence of parasitism ± SE by T. minutum on codling moth sentinel eggs in the experimental apple orchard (ANOVA, P < 0.05)
Combined mortality of C. pomonella by T. minutum and CpGV in the orchard
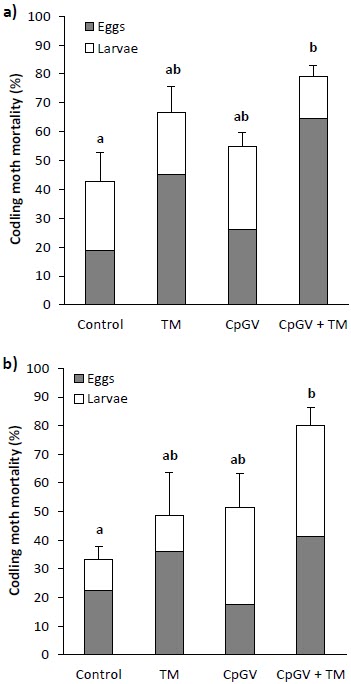
In the first year, combined mortality in the CpGV + TM treatment was 1.85 times higher and statistically different than in the control (F(3,12) = 4.05, P = 0.0201) (Fig. 3a). In year two, combined mortality in the control (33.2%) was significantly lower than combined mortality in the CpGV + TM treatment (80.2%) (F(3,12) = 5.21, P = 0.003). However, there was no significant difference between the TM treatment, the CpGV treatment and the CpGV + TM treatment in both years (Fig. 3).
Figure 3
Combined mortality ± SE of codling moth caused by T. minutum on eggs and by CpGV on larvae in all treatments in the experimental orchard in summer year 1 (a) and summer year 2 (b) (ANOVA, P < 0.05)
DISCUSSION
Laboratory bioassays: compatibility of biocontrol agents
Parasitism rate on codling moth eggs previously treated with CpGV at three concentrations was lower than in the control and the sugar and milk treatments. We believe that T. minutum females detected the presence of the virus and/or adjuvants found in the formulations on the codling moth eggs and rejected them as quality hosts. Other studies have also found that parasitism of Trichogramma species has been reduced as a result of females being in contact with host eggs treated with bioinsecticides (Broglio-Micheletti et al. 2006; Sidi et al. 2012
). For example, parasitism by T. pretiosum on Anagasta kuehniella Zeller (Lepidoptera: Pyralidae) eggs that were previously sprayed with Metarhizium anisopliae (Metsch.) (Hypocreales) was reduced by 47% when compared to the control (Potrich et al. 2017). Trichogramma spp. are known to evaluate the quality of host eggs, their size, age, colour and the host species (Lobdel et al. 2005; Monje et al. 1999). This discrimination by T. minutum females is a desired trait for the combined use of biocontrol agents. It may allow females to avoid virus-contaminated eggs, parasitizing uncontaminated eggs and thus increasing the host mortality and the impact on codling moth population. In other words, female parasitoids can avoid CpGV-contaminated eggs, which is explained by lower rates of parasitism on virus-contaminated eggs. Nevertheless, Khan (2017) found that CpGV did not affect oviposition and parasitism by T. chilonis at several concentrations, 12.5X, 6.25X, 2.5X, and 1.25X. This difference could be explained by unlike oviposition behaviours between T. minutum on Cydiapomonella eggs in Sitotrogacerealella (Khan 2017).
Most larvae of koinobiont endoparasitoids are affected by simultaneous virus infections occurring in the host (Nakai 2009). However, Trichogramma spp. are idiobiont endoparasitoids (Querino et al. 2009) and the outcome of the interaction virus-parasitoid might not be affected by the metabolic changes of the host facing the virus, as it can be the case of koinobiont endoparasitoids. Furthermore, CpGV is known to be harmless to non-target insects (Falcon et al. 1968).
Orchard trials
The results confirmed partly our original hypothesis since CpGV and T. minutum are compatible when used together in the orchard, but we did not observe any additive effect on codling moth mortality.
Regarding mortality of larvae in the orchard, it is important to note that we assessed codling moth mortality after the neonate larvae ingested virus particles from the chorion. Larvae can also be exposed to the virus while browsing on foliage or penetrating apple (Ballard et al. 2000b, Lacey et al. 2008). Consequently, we might have underestimated larval mortality.
Incidence of parasitism
Unlike laboratory bioassays, the presence of the virus on eggs did not reduce the incidence of parasitism by T. minutum in the orchard. Different environmental conditions mostly UV inactivation of CpGV by the sun (Wu et al. 2015) could explain the difference between laboratory experiments and orchard trials. Probably, parasitoid females encountered less viral bodies contained on host eggs on apple leaves than on those in glass tubes, especially after two days, as it is known that half-lives on foliage vary between 2 and 3 d (Jaques et al. 1987). This finding is particularly relevant for codling moth control because it suggests that both biocontrol agents are compatible to be used together in the orchard.
In the orchard, an interesting finding that was not directly related with our hypothesis testing was the high incidence of parasitism found in the control, as well as in the CpGV treatment, where no T. minutum had been released. Even though codling moth sentinel eggs could have been parasitized by indigenous Trichogramma, chemical insecticides and fungicides were used in most areas of the experimental orchard (excluding our study area) and the indigenous population of Trichogramma was probably low, as the literature indicates detrimental effects of pesticides on the Trichogrammatidae, such as high mortality, low parasitism rates, low survival time and reduced progeny (Brunner 1996; Grützmacher et al. 2004; Vieira et al. 2001). This indicates that most sentinel eggs were probably parasitized by T. minutum emerged from trichocards in nearby treated plots.
Combined mortality of C. pomonella by T. minutum and CpGV in the orchard
Since the results of the combined effect of biocontrol agents in both years of the study show similar trends (Fig. 3), is unlikely that differences between Madex and Virosoft CP4® CpGV isolates (Lacey et al. 2008; Vincent et al. 2007) could have affected the outcome of codling moth mortality.
The combined use of T. minutum and CpGV in the orchard was not detrimental for parasitism activity. Based on observed discrimination of CpGV-contaminated codling moth eggs by female parasitoids in laboratory bioassays, it is possible that discrimination also occurred during the first days following virus application in the orchard. However, as the virus degraded because of exposure to biotic factors (mainly UV radiation), discrimination of eggs decreased thereafter. A detailed study about discrimination of infected eggs by female parasitoids after CpGV treatments is necessary to fully evaluate the advantages for producers of using both biocontrol agents at the same time.
Nevertheless, as our statistical analyses revealed no difference between treatments for combined mortality, we can assume that no significant antagonistic or synergistic interaction changed the combined efficiency of the two biocontrol agents. CpGV and T. minutum have different modes of action and the targeted stages of development of the pest are not the same. Since CpGV and T. minutum are compatible, when used at the same time, if one biocontrol agent fails, the other can compensate and increase the chances of success throughout the growing season when the host is present. Although costs of biocontrol products are still greater than conventional pesticides, high levels of mortality in the orchard trials suggest that using CpGV and T. minutum together has potential to become economically acceptable to apple growers. Mass production of biocontrol agents could make this option economically sound in the future. Additionally, studies of economic sustainability on such strategy, as part of insect pest management programs should be conducted as well.
Our study is the first to examine interactions between T. minutum and CpGV, and even considering that more research is needed to fully understand this system, both agents used in combination hold considerable potential to be used in organic as well as in integrated fruit production orchards.
Appendices
Acknowledgements
We thank the Conseil pour le développement de l’agriculture du Québec (CDAQ) and Nova Scotia’s ACAAF Council for funding. We thank Franz Vanoosthuyse and Francine Pelletier for their support on the field and for assistance in data analyses. We are grateful to Anik P. Leboeuf, Nicolas Marie, IRDA’s research team and UQAM students for their help in the field. We thank Jocelyn Tardif for great advice and collaboration during experiments. We also thank M. Martin Andermatt for his support in the project and for supplying the Madex® formulation.
REFERENCES
- Ansari, M.A., F.A. Shah, and T.M. Butt. 2010. The entomopathogenic nematode Steinernema kraussei and Metarhizium anisopliae work synergistically in controlling overwintering larvae of the black vine weevil, Otiorhynchus sulcatus, in strawberry growbags. Biocontrol Sci. Technol. 20: 99-105.
- Arthurs, S.P., and L.A. Lacey. 2004. Field evaluation of commercial formulations of the codling moth granulovirus: persistence of activity and success of seasonal appli-cations against natural infestations of codling moth in Pacific Northwest apple orchards. Biol. Control 31: 388-397.
- Arthurs, S.P., L.A. Lacey, and R. Fritts, Jr. 2005. Optimizing use of codling moth granulovirus: effects of application rate and spraying frequency on control of codling moth larvae in Pacific Northwest apple orchards. J. Econ. Entomol. 98: 1459-1468.
- Aubry, O. 2008. Lutte attracticide et lâchers inondatifs de trichogrammes contre le carpocapse de la pomme, Cydia pomonella (Lepidoptera : Tortricidae). Université du Québec à Montréal, Canada, 114 pp. Available online [https://archipel.uqam.ca/1052/].
- Ballard, J., D.J. Ellis, and C.C. Payne. 2000a. The role of formulation additives in increasing the potency of Cydia pomonella granulovirus for codling moth larvae, in laboratory and field experiments. Biocontrol Sci. Technol. 10: 627-640.
- Ballard, J., D.J. Ellis, and C.C. Payne. 2000b. Uptake of granulovirus from the surface of apples and leaves by first instar larvae of the codling moth Cydiapomonella L. (Lepidoptera: Olethreutidae). Biocontrol Sci. Technol. 10: 617-625.
- Barnes, M.M. 1991. Tortricids in pome and stone fruits. Codling moth occurrence, host race formation, and damage. Pages 313-328 in L.P.S. van der Geest, and H.H. Evenhuis (eds.), Tortricid pests. Their biology, natural enemies and control. Elsevier, Amsterdam, Netherlands.
- Blomefield, T.L., and J.H. Giliomee. 2012. Fecundity and mortality of codling moth, Cydia pomonella (L.) (Lepidoptera: Tortricidae), under field conditions in South Africa. Afr. Entomol. 20: 316-324.
- Botto, E., and P. Glaz. 2010. Potential for controlling codling moth Cydia pomonella (Linnaeus) (Lepidoptera: Tortricidae) in Argentina using the sterile insect technique and egg parasitoids. J. Appl. Entomol. 134: 251-260. doi:10.1111/ j.1439-0418.2009.01389.x
- Broglio-Micheletti, S.M.F., A.J.N. dos Santos, and J.L. Pereira-Barros. 2006. Action of some phytossanitary products for Trichogramma galloi Zucchi, 1988 (Hymenoptera: Trichogrammatidae). Ciênc. Agrotec. 30: 1051-1055.
- Brunner, J.F. 1996. Discovery of Colpoclypeus florus (Walker) (Hymenoptera: Eulophidae) in apple orchards of Washington. Pan-Pac. Entomol. 72: 5-12.
- Chouinard, G., A. Firlej, and D. Cormier. 2016. Going beyond sprays and killing agents: exclusion, sterilization and disruption for insect pest control in pome and stone fruit orchards. Sci. Hortic. 208: 13-27. doi:10.1016/j.scienta. 2016.03.014
- Cormier, D., P. Cabrera, F. Vanoosthuyse, M. Fournier, and É. Lucas. 2017. Effects of two reduced-risk insecticides on the egg parasitoid Trichogramma minutum in apple orchards. IOBC-WPRS Bull. 123: 110-114.
- Cormier, D., G. Chouinard, F. Vanoosthuyse, F. Pelletier, S. Bellerose, G. Bourgeois, D. Plouffe, and R. Joannin. 2015. A phenology model for codling moth management in Quebec apple orchards. Acta Hortic. 1068: 51-56. doi:10.17660/Acta Hortic.2015.1068.5
- Cossentine, J.E., and L.B.M. Jensen. 2000. Releases of Trichogramma platneri (Hymenoptera: Trichogrammatidae) in apple orchards under a sterile codling moth release program. Biol. Control 18: 179-186. doi:10.1006/bcon. 2000.0828
- Denoth, M., L. Frid, and J.H. Myers. 2002. Multiple agents in biological control: improving the odds? Biol. Control 24: 20-30. doi:10.1016/S1049-9644(02)00002-6
- Falcon, L.A., W.R. Kane, and R.S. Bethell. 1968. Preliminary evaluation of a granulosis virus for control of the codling moth. J. Econ. Entomol. 61: 1208-1213. doi:10.1093/jee/ 61.5.1208
- Furlong, M.J., and J.K. Pell. 2000. Conflicts between a fungal entomopathogen, Zoophthora radicans, and two larval parasitoids of the diamondback moth. J. Invertebr. Pathol. 76: 85-94.
- Gonzalez, F., C. Tkaczuk, M.M. Dinu, Ż. Fiedler, S. Vidal, E. Zchori-Fein, and G.J. Messelink. 2016. New opportunities for the integration of microorganisms into biological pest control systems in greenhouse crops. J. Pest Sci. 89: 295-311.
- Grützmacher, A.D., O. Zimmermann, A. Yousef, and S.A. Hassan. 2004. The side-effects of pesticides used in integrated production of peaches in Brazil on the egg parasitoid Trichogramma cacoeciae Marchal (Hym., Trichogrammatidae). J. Appl. Entomol. 128: 377-383.
- Jaques, R.P., J.M. Hardman, J.E. Laing, R.F. Smith, and E. Bent. 1994. Orchard trials in Canada on control of Cydia pomonella (Lep: Tortricidae) by granulosis virus. Entomophaga 39: 281-292.
- Jaques, R.P., J.E. Laing, D.R. Laing, and D.S.K. Yu. 1987. Effectiveness and persistence of the granulovirus of the codling moth Cydia pomonella L. (Lepidoptera: Olethreutidae) on apple. Can. Entomol. 119: 1063-1067. doi:10.4039/ Ent1191063-12
- Khan, M.A. 2017. Effect of selected baculoviruses on oviposition preference by Trichogramma chilonis (Trichogrammatidae: Hymenoptera). J. King Saud Univ. Sci. 29: 214-220. doi:10. 1016/j.jksus.2016.06.002
- Kienzle, J., O. Zimmermann, B. Wührer, P. Triloff, J. Morhard, E. Landsgesell, and C.P.W. Zebitz. 2012. New species and new methods of application – a new chance for Trichogramma in codling moth control? Pages 317-321 in Proceedings of the Ecofruit. 15th international conference on organic fruit-growing. Hohenheim, Germany.
- Koppenhöfer, A.M., and H.K. Kaya. 1997. Additive and synergistic interaction between entomopathogenic nematodes and Bacillus thuringiensis for scarab grub control. Biol. Control 8: 131-137. doi:10.1006/bcon.1996. 0498
- Lacey, L.A., S. Arthurs, A. Knight, K. Becker, and H. Headrick. 2004. Efficacy of codling moth granulovirus: effect of adjuvants on persistence of activity and comparison with other larvicides in a Pacific Northwest apple orchard. J. Entomol. Sci. 39: 500-513.
- Lacey, L.A., S.P. Arthurs, and H. Headrick. 2005. Comparative activity of the codling moth granulovirus against Grapholitamolesta and Cydiapomonella (Lepidoptera: Tortricidae). J. Entomol. Soc. Brit. Columbia 102: 79-80.
- Lacey, L.A., D. Thomson, C. Vincent, and S.P. Arthurs. 2008. Codling moth granulovirus: a comprehensive review. Biocontrol Sci. Technol. 18: 639-663. doi:10.1080/09583 150802267046
- Lacey, L.A., and T.R. Unruh. 2005. Biological control of codling moth (Cydia pomonella, Lepidoptera: Tortricidae) and its role in integrated pest management, with emphasis on entomopathogens. Vedalia 12: 33-60.
- Lobdell, C.E., T.-H.Yong, and M.P. Hoffmann. 2005. Host color preferences and short-range searching behavior of the egg parasitoid Trichogramma ostriniae. Entomol. Exp. Appl. 116: 127-134. doi:10.1111/j.1570-7458.2005. 00306.x
- Miller, J.R., and L.J. Gut. 2015. Mating disruption for the 21st century: matching technology with mechanism. Environ. Entomol. 44: 427-453.
- Mills, N., C. Pickel, S. Mansfield, S. McDougall, R. Buchner, J. Caprile, J.P. Edstrom, R.B. Elkins, J.K. Hasey, K. Kelley, B. Krueger, B. Olson, and R. Stocker. 2000. Mass releases of wasps can reduce damage from codling moth. Calif. Agr. 54: 22-25.
- Moffitt, H.R., and A.K. Burditt, Jr. 1989. Low-temperature storage as a postharvest treatment for codling moth (Lepidoptera: Tortricidae) eggs on apple. J. Econ. Entomol. 82: 1679-1681.
- Monje, J.C., C.P.W. Zebitz, and B. Ohnesorge. 1999. Host and host age preference of Trichogramma galloi and T. pretiosum (Hymenoptera: Trichogrammatidae) reared on different hosts. J. Econ. Entomol. 92: 97-103. doi:10. 1093/jee/92.1.97
- Morin, Y., D. Cormier, and G. Chouinard. 2017. Le carpocapse de la pomme. Fiche 76 Guide de référence en production fruitière intégrée 2016. Available online [https://reseaupommier.irda.qc.ca/?p=6431] (Accessed in October 2019).
- Nakai, M. 2009. Biological control of tortricidae in tea fields in Japan using insect viruses and parasitoids. Virol. Sin. 24: 323-332.
- Potrich, M., L.F.A. Alves, E.R. Lozano, A.K. Bonini, and P.M.O.J. Neves. 2017. Potential side effects of the entomopathogenic fungus Metarhizium anisopliae on the egg parasitoid Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) under controlled conditions. J. Econ. Entomol. 110: 2318-2324.
- Querino, R.B., R.A. Zucchi, and J.D. Pinto. 2009. Systematics of the Trichogrammatidae (Hymenoptera: Chalcidoidea) with a focus on the genera attacking Lepidoptera. Pages 191-218 in L.F. Cônsoli, J.R.P. Parra, and R.A. Zucchi (eds.), Egg parasitoids in agroecosystems with emphasis on Trichogramma. Springer.
- Rännbäck, L.-M., B. Cotes, P. Anderson, B. Rämert, and N.V. Meyling. 2015. Mortality risk from entomopathogenic fungi affects oviposition behavior in the parasitoid wasp Trybliographa rapae. J. Invertebr. Pathol. 124: 78-86. doi: 10.1016/j.jip.2014.11.003
- SAS Institute. 2018. Non parametric tests. Available online [https://www.jmp.com/support/help/14/nonparametric-tests.shtml] (Accessed on December 2019).
- Schmitt, A., I.L. Bisutti, E. Ladurner, M. Benuzzi, B. Sauphanor, J. Kienzle, D. Zingg, K. Undorf-Spahn, E. Fritsch, J. Huber, and J.A. Jehle. 2013. The occurrence and distribution of resistance of codling moth to Cydia pomonella granulovirus in Europe. J. Appl. Entomol. 137: 641-649. doi:10.1111/ jen.12046
- Sidi, M.B., M.T. Islam, Y. Ibrahim, and D. Omar. 2012. Effect of insecticide residue and spray volume application of azadirachtin and rotenone on Trichogramma papilionis (Hymenoptera: Trichogrammatidae). Int. J. Agric. Biol. 14: 805-810.
- Sigsgaard, L., A. Herz, M. Korsgaard, and B. Wührer. 2017. Mass release of Trichogramma evanescens and T. cacoeciae can reduce damage by the apple codling moth Cydia pomonella in organic orchards under pheromone disruption. Insects 8: 41.
- Snyder, W.E., and A.R. Ives. 2003. Interactions between specialist and generalist natural enemies: parasitoids, predators, and pea aphid biocontrol. Ecology 84: 91-107. doi:10.1890/0012-9658(2003)084[0091:IBSAGN]2.0.CO;2
- Sokal, R.R., and F.J. Rohlf. 1995. Biometry: the principles and practice of statistics in biological research. Freeman, New York, NY, USA. 887 pp.
- Vieira, A., L. Oliveira, and P. Garcia. 2001. Effects of conventional pesticides on the preimaginal developmental stages and on adults of Trichogramma cordubensis (Hymenoptera: Trichogrammatidae). Biocontrol Sci. Technol. 11: 527-534.
- Vincent, C., M. Andermatt, and J. Valéro. 2007. Madex and Virosoft CP4, viral biopesticides for codling moth control. Pages 336-343 in C. Vincent, M.S. Goettel, and G. Lazarovits (eds.), Biological control: a global perspective. Case Histories from around the world. CABI Publishing, Wallingford, UK.
- Witzgall, P., L. Stelinski, L. Gut, and D. Thomson. 2008. Codling moth management and chemical ecology. Annu. Rev. Entomol. 53: 503-522. doi:10.1146/annurev.ento. 53.103106.093323
- Wu, Z.-W., J.-B. Fan, H. Yu, D. Wang, and Y.-L. Zhang. 2015. Ultraviolet protection of the Cydia pomonella granulovirus using zinc oxide and titanium dioxide. Biocontrol Sci. Technol. 25: 97-107.
- Yu, D.S.K., E.A.C. Hagley, and J.E. Laing. 1984. Biology of Trichogramma minutum Riley collected from apples in Southern Ontario. Environ. Entomol. 13: 1324-1329. doi: 10.1093/ee/13.5.1324
- Zhang, J.-J., B.-Z. Ren, X.-H. Yuan, L.-S. Zang, C.-C. Ruan, G.-Z. Sun, and X.-W. Shao. 2014. Effects of host-egg ages on host selection and suitability of four Chinese Trichogramma species, egg parasitoids of the rice striped stem borer, Chilo suppressalis. BioControl 59: 159-166. doi:10.1007/ s10526-013-9557-4
- Zimmermann, G. 2007. Review on safety of the entomo-pathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Sci. Technol. 17: 553-596. doi:10. 1080/09583150701309006
List of figures
Figure 1
Effect of CpGV on parasitism of codling moth eggs by T. minutum in the laboratory. a) Rate of parasitized eggs ± SE. b) Average number of T. minutum offspring emerged per parasitized codling moth egg ± SE (ANOVA, P < 0.05)
Figure 2
Incidence of parasitism ± SE by T. minutum on codling moth sentinel eggs in the experimental apple orchard (ANOVA, P < 0.05)
Figure 3
Combined mortality ± SE of codling moth caused by T. minutum on eggs and by CpGV on larvae in all treatments in the experimental orchard in summer year 1 (a) and summer year 2 (b) (ANOVA, P < 0.05)